Simultaneous cardiac and cerebral infarcts in a patient with hip fracture and previously unknown patent foramen ovale: management between Scylla and Charybdis
Alexander Fisher 1,2, Emily Walsh 1*
1Department of Geriatric Medicine. The Canberra Hospital, Canberra, Australian Capital Territory, Australia.
2Australian National University Medical School, Canberra, Australian Capital Territory, Australia
*Corresponding author
*Dr Emily Walsh, Department of Geriatric Medicine, The Canberra Hospital, Yamba Drive, Garran ACT, m 2605, Australia.
DOI: 10.55920/JCRMHS.2023.03.001133
Figure 1. CT scan (a) and Xray (b) showing left basicervical neck of femur fracture.
On arrival to his home, the ambulance paramedic team noted that the patient was conscious, alert, well perfused with Glasgow Coma Scale (GCS) of 15, but had blue tinged lips, respiratory rate of 30/minute and required 5 L/min of supplemental oxygen (via nasal prong) to keep oxygen saturation (O2 sat) ≥92%. On the patient’s arrival at the hospital, although he denied any shortness of breath or chest pain, marked hypoxaemia was documented (pO2 of 51 mmHg on FiO2 of 44%, pH 7.45, PaCO2 36 mmHg, and O2 sat 84%); he was put on oxygen via high flow nasal prongs (HFNP) FiO2 30% at 30 L/min to maintain saturation ≥ 92% (pO2 increased to only 69 mmHg). On examination, he was calm, showing no signs of respiratory distress. The blood pressure (BP) was 145/95 mmHg, heart rate 95 beats per minute (bpm), respiratory rate 16/minute, temperature 36.9oC, GCS 15 (E4V5M6), body mass index (BMI) 27.3 kg/m2. Pulmonary auscultation revealed crepitations bilaterally up to the middle zones, and the jugular venous pressure (JVP) was elevated (9 cm). Otherwise, his cardiovascular and abdominal examination was unremarkable. Neurological examination did not reveal focal motor or sensory deficit, facial droop, signs of meningeal irritation, tremor of extremities; the flexor plantar responses and deep tendon reflexes were normal. No skin rashes, petechia and no significant lymphadenopathy was noted. On admission, a twelve-lead electrocardiogram (ECG) showed sinus tachycardia (100 bpm) without specific ST-T segment changes but with Q waves in leads III and aVF (Figure 2a). Computed tomography (CT) of the brain without contrast was performed within 90 minutes of arrival, it demonstrated chronic white matter ischaemic changes (microangiopathy), mild focal gliosis in the inferior aspect of the right frontal lobe (consistent with old infarct), global cortical atrophy with corresponding sulcul widening and no features of acute intracranial pathology (Figure 3a). The chest X-ray showed pulmonary venous congestion (Figure 4). The clinical and radiological signs of acute pulmonary oedema (due to possible fluid overload) were interpreted as the main explanation of hypoxia and requirement of high oxygen supplementation (periodically up to HFNF FiO2 40% at 40L/minute). Blood tests results including full blood count (haemoglobin 155 g/L, red blood cells 4.84 x1012/L, haematocrit 0.46), liver enzyme levels, urea, creatinine, electrolytes, thyroid function, parathyroid hormone, vitamin D, vitamin B12, folic acid, indices of iron metabolism were all within reference ranges; urinalysis and urine culture ruled out urinary tract infection and coronavirus nucleic acid testing (PCR) was negative. However, four immuno-inflammatory markers were abnormal: the neutrophil lymphocyte ratio (NLR) was 21.8 (normal value <3), lymphocyte monocyte ratio (LMR) was 0.93 (normal value >1.1), the systemic immune inflammation index (SII = platelet × NLR) was 3248.2 (normal value <1000.0) and CRP was 96.9 mg/L (normal value <6 mg/L) indicating presence of an inflammatory process.
Figure 2. ECGs: (a) on admission (sinus tachycardia at a rate of 100 bpm, old Q waves in leads III and aVF, and poor R wave progression); and (b) on the next day when cardiac troponin I level was 1093 ng/L (NSTEMI).
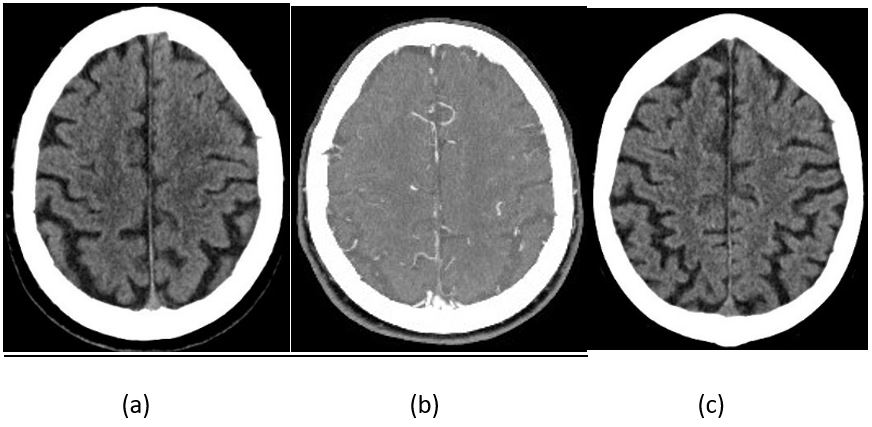
Figure 3. CT brain: (a) on admission (did not show any acute ischemia or haemorrhage); (b) CT angiogram and perfusion (did not show acute ischemia, haemorrhage, or vessel occlusion); and (c) on day eight of admission (did not show any acute ischemia or haemorrhage).
Figure 4. Anteroposterior chest X-ray on admission showing pulmonary venous congestion.
On the second admission day (25 hours post arrival to the hospital) he reported inability to move his arms. His physical examination was positive for bilateral proximal arm weakness, worse on the left (grade 1-2/5); he was sleepy, confused, at times not fully oriented to place, and uncooperative, GCS was 13 (E3V4M6) and his BP was 200/105 mmHg. Although he denied chest pain, the high sensitivity cardiac troponin I (hs-cTrI) level was 1093 ng/L (normal value <26 ng/L) and 852 ng/L 4 hours later consistent with a non-ST elevation myocardial infarction (NSTEMI) (Figure 2b). He maintained an O2 sat of 85-89%, despite high-flow oxygen nasal cannula. The second CT of the brain and cervical spine did not show any acute intracranial ischemic or haemorrhagic changes, cervical spine abnormality or mass lesion (Figure 3b). The cardiology, neurology and stroke teams were consulted. Following cardiologist’s advice, he was treated with frusemide (20 mg iv, then 40 mg daily), glyceryl trinitrate patch (5 mg/hour, removed when systolic BP ≤160 mmHg), heparin infusion (for 48 hours), aspirin (300 mg stat, followed by aspirin 100 mg daily); coronary angiogram was not performed due to patient’s comorbid status, and clopidogrel was not commenced as he required urgent surgery for the HF.
On the third admission day, the left sided arm weakness persisted, GCS dropped to 8 (E1V2M5), the National Institutes of Health Stroke Scale (NIHSS) score was 23, temperature 38°C, the C-reactive protein (CRP) level raised to 103.1 mg/L, neutrophil count to 10.2 x109/L (normal range: 1.8-7.5 x109/L) without leucocytosis 11.6 x109/L (normal range: 4.0-11.0 x109/L), and the NLR was 17.9, LMR was 0.78 and SII was 9554. The CT pulmonary angiogram showed no acute pulmonary embolism (PE), consolidation or pneumothorax, no aortic dissection, but dependent ground-glass opacities and intra-lobular septal thickening within both lungs, most predominantly within the right lung (Figure 5). Further septic workup revealed Streptococcus agalactiae (Group B) in the urine (the patient had an indwelling urinary catheter since admission). The hypoactive delirium and fluctuating GCS were thought to be secondary to evolving infection and NSTEMI. The patient was commenced on ceftriaxone (1 g iv daily) and azithromycin (500 mg daily) to treat the urinary tract infection and possible aspiration-related lung injury.
Figure 5. CT pulmonary angiogram (no acute pulmonary embolism, dependent ground glass opacities and intralobular septal thickening, most marked within right lung base, in keeping with pulmonary oedema).
On the fourth admission day, the patient had an episode of left facial droop, intermittent left arm twitch and GCS of 8 (E4V3M1) which improved spontaneously within 20 minutes to 14 (E4V4M6); a subtle left facial droop remained and focal myoclonic activity on the left side of the face were noted. A repeated CT scan (fourth) of the brain did show no acute cerebral ischemia or intracranial haemorrhage. Contrast enhanced CT angiogram from aortic arch to circle of Willis revealed no large vessel occlusion involving the circle of Willis with good contrast opacification of the carotid and vertebral arteries bilaterally; there were calcified atheromas at both carotid bulbs and proximal internal carotid arteries associated with 30% stenosis on the right and 35% stenosis on the left. However, on the next day (5th day of admission) his brain magnetic resonance imaging (MRI) disclosed widespread infarcts in multiple vascular territories (Figure 6)—frontal lobes, including the centrum semiovale, parietal and occipital lobes, involving the grey matter and grey-white matter junction, and similar foci in the right cerebellar hemisphere; a small focus of microhaemorrhage was present in grey matter of the right parietal zone; there were also periventricular T2/FLAIR hyperintensities indicating chronic small vessel ischaemic changes.
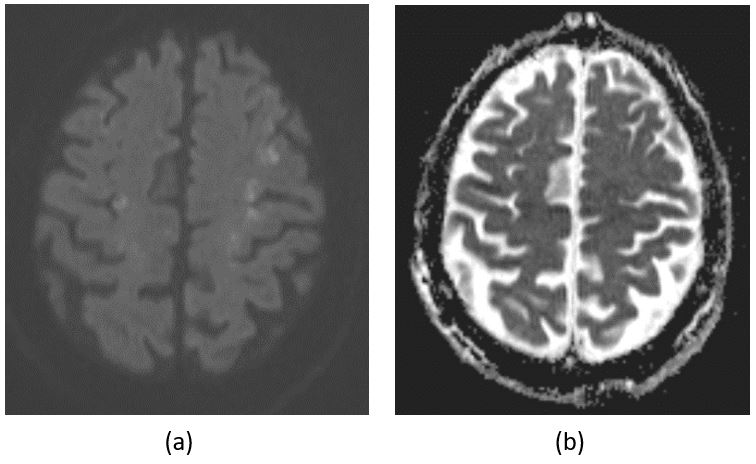
Figure 6. Axial diffusion-weighted magnetic resonance imaging showing acute multi-territorial infarcts (in an internal boarder zone distribution) involving the frontal, parietal, occipital lobes, and the right cerebellum (a), with corresponding apparent diffusion coefficient hypointensity in frontal lobe subcortical white matter (b).
Extensive watershed infarcts, which were not visible on previous CT scans, and the patient’s persisted hypoxaemia (disproportionate to the APO, atelectasis, AMI, and infection) in the absence of a pulmonary embolism, prompted further investigation; an anatomic shunt was considered. Cardiac and lower extremities ultrasound and Holter monitoring were performed. No signs of deep vein thrombosis of the lower extremities were detected. Holter monitoring that recorded about 25 hours showed dominant sinus rhythm, infrequent isolated supraventricular ectopic beats with 7 couplets and 9 non-sustained supraventricular tachycardia events (longest 14 beats at 129 bpm and fastest 4 beats at 145 bpm) and infrequent isolated ventricular ectopic beats with 2 triplets and 1 non-sustained wide complex tachycardia lasting 29 beats at 182 bpm; there was no evidence of atrial fibrillation (AF), flutter or symptomatic arrhythmia. Transthoracic echocardiography discovered a dilated left atrium, normal right atrial size, aneurysmal and mobile inter-atrial septum with evidence of right-to-left shunt across the interatrial septum (on colour Doppler) consistent with PFO and atrial septal defect; left and right ventricular cavity sizes and systolic function were normal, ejection fraction (EF) 59%, and no obvious regional wall motion abnormalities; there was a mildly dilated aortic root (42 mm), moderately dilated proximal ascending aorta (46 mm), mild aortic, tricuspid and pulmonary regurgitation; no thrombi or other sources of emboli were detected (Figure 7a). The echocardiography examination with saline contrast bubble infusion showed an aneurysmal and mobile interatrial septum and confirmed the PFO with a large right-to-left shunt (Figure 7b).
Figure 7. Transthoracic echocardiograms, (a) with colour duplex showing the PFO; and (b) showing a positive “bubble” study confirming a right-to-left shunt across a PFO, agitated saline is injected intravenously and bubbled are seen opacifying the right atrium.
The patient underwent left hip hemiarthroplasty on the fifth admission day (6 hours after he completed 48 hours of heparin infusion) under general anaesthesia. A left suprainguinal fascia iliaca block (prior to induction) was followed by intravenous propofol (2 mg/kg), fentanyl (200 mcg) and rocuronium (0.8 mg/kg); tranexamic acid (1g iv) was given for surgical haemostasis. Throughout the procedure general anaesthesia was maintained with sevoflurane (1.8% to 2.2%) in O2. Intraoperatively his BP readings ranged between 90/50 mmHg and 170/90 mmHg; to maintain mean arterial pressure (MAP) of ≥ 80mmHg metaraminol was administered. At the end of the procedure, tranexamic acid (6 g) and vancomycin (2g) were flushed into the surgical wound. The patient was extubated and returned to the ward; he tolerated oxygen supplementation using nasal cannula. Postoperative haemoglobin and haematocrit were 125 g/L and 0.37, respectively (dropped from preoperative 151 g/L and 0.40, respectively). Preoperatively hs-cTrI was 140 ng/L, it dropped to 81 ng/L two days later.
Thrombolytic therapy for myocardial infarction was considered to be of high risk for developing haemorrhage (both intracranial and from the surgical wound). Following the advice by the neurologist and cardiologist, prophylactic anticoagulation with low molecular weight heparin (LMWH, enoxaparin 40 mg subcutaneously) was started while aspirin, low density lipoprotein cholesterol lowering therapy with ezetimibe, esomeprazole and antibiotics were continued. Plans were made for percutaneous closure of the PFO a week later.
On the second postoperative day he developed a drop of GCS to 3 (E1V1M1) for about 30 minutes, was unresponsive but no tonic or clonic features were observed; the GCS subsequently improved to 13 (E4M5V4). The fifth CT brain scan did not show any acute ischemic or haemorrhagic changes (Figure 3c). With ongoing neurological presentation due to cerebral infarcts an absence seizure was suspected, the patient was commenced on levetiracetam (1 g twice daily). Over the next two weeks the patient remained stable, and an overall improvement was observed, consciousness restored, he had better verbal responses, but maintained the left-sided upper and lower extremity weakness with inability to resist gravity (3/5) and moderate-severe unsteadiness. On the fifth postoperative day, the levels of hs-cTrI and inflammatory markers were in the normal range. Unfortunately, on the third postoperative week his dysarthria and dysphasia increased, he developed dysphagia (mild-moderate) and aspiration-related lung injury associated with elevated inflammatory markers (CRP raised from 38.2 mg/L on the sixth postoperative day to 201.0 mg/L on the 21st day); he required feeding via a nasogastric tube.
A week later he was diagnosed with hospital acquired COVID-19 infection (treated with remdesivir and prednisolone) and two days later developed upper gastrointestinal bleeding, anaemia (haemoglobin 102g/L, haematocrit 0.31) and severe hypotension (70/45 mmHg) requiring ICU admission. Despite aggressive medical management including red blood cell transfusion, proton pump inhibitor pantoprazole (40 mg iv every 8 hours), discontinuation of all anti-thrombotic and antiplatelet agents he continued to decline; the status was discussed with the family, which decided on comfort care, and he died the next day.
The discharge diagnosis was simultaneous acute myocardial infarction and acute cerebral infarctions due to PFO and atrial septal defect in a patient with left hip fracture and perioperative urinary tract infection, postoperatively complicated with aspiration-related lung injury, COVID-19 infection, and fatal upper gastrointestinal bleeding.
- Fisher, A.A.; Southcott, E.N.; Goh, S.L.; Srikusalanukul.; Hickman, P.E.; Davis, M.W.; Potter, J.M.; Budge, M.M.; Smith, P.N. Elevated serum cardiac troponin I in older patients with hip fracture: incidence and prognostic significance. Arch Orthop Trauma Surg 2008, 128, 1073-9. doi: 10.1007/s00402-007-0554-x. PMID: 18193436.
- Dawson-Bowling, S.; Chettiar, K.; Cottam, H.; Worth, R.; Forder, J.; Fitzgerald-O’Connor, I.; Walker, D.; Apthorp, H. Troponin T as a predictive marker of morbidity in patients with fractured neck of femur. Injury 2008, 39, 775-80. doi: 10.1016/j.injury.2008.01.025. PMID: 18407276.
- Puelacher, C.; Lurati Buse, G.; Seeberger, D.; Sazgary, L.; Marbot, S.; Lampart, A.; Espinola, J.; Kindler, C.; Hammerer, A.; Seeberger, E.; Strebel, I.; Wildi, K.; Twerenbold, R.; du Fay de Lavallaz, J.; Steiner, L.; Gurke, L.; Breidthardt, T.; Rentsch, K.; Buser, A.; Gualandro, D.M.; Osswald, S.; Mueller, C.; BASEL-PMI Investigators. Perioperative myocardial injury after noncardiac surgery: incidence, mortality and characterisation. Circulation 2018, 137, 1221-1232. doi: 10.1161/CIRCULATIONAHA.117.030114. PMID: 29203498.
- Kim, B.S.; Kim, T.H.; Oh, J.H.; Kwon, C.H.; Kim, S.H.; Kim, H.J.; Hwang, H.K.; Chung, S.M. Association between preoperative high sensitive troponin I levels and cardiovascular events after hip fracture surgery in the elderly. J Geriatr Cardiol 2018, 15, 215-221. doi: 10.11909/j.issn.1671-5411.2018.03.002. PMID: 29721000.
- Lowe, M.J.; Lightfoot, N.J. The prognostic implication of perioperative cardiac enzyme elevation in patients with fractured neck of femur: a systematic review and meta-analysis. Injury 2020, 51, 164-173. doi: 10.1016/j.injury.2019.12.012. PMID: 31879176.
- Gao, L.; Chen, L.; He, J.; Wang, B.; Liu, C.; Wang, R.; Fan, L.; Cheng, R. Perioperative myocardial injury/infarction after non-cardiac surgery in elderly patients. Front Cardiovasc Med 2022, 9, 910879. doi: 10.3389/fcvm.2022.910879. PMID: 35665266.
- Rostagno, C.; Cammilli, A.; Di Cristo, A.; Polidori, G.L.; Ranalli, C.; Cartei, A.; Buzzi, R.; Prisco, D. Acute coronary syndromes with significant troponin increase in patients with hip fracture prior to surgical repair: differential diagnosis and clinical implications. Intern Emerg Med 2016, 11, 219-24. doi: 10.1007/s11739-015-1348-8. PMID: 26563767.
- Sukernik, M.R.; Mets, B.; Bennett-Guerrero, E. Patent foramen ovale and its significance in the perioperative period. Anesth Analg 2001, 93, 1137-46. doi: 10.1097/00000539-200111000-00015. PMID: 11682383.
- Rajan, G.R.; Intractable intraoperative hypoxemia secondary to pulmonary embolism in the presence of undiagnosed patent foramen ovale. J Clin Anesth 2007, 19, 374-7. doi: 10.1016/j.jclinane.2006.09.011. PMID: 17869991
- Karttunen, V.; Hiltunen, L.; Rasi, V.; Vahtera, E.; Hillbom, M. Factor V Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul Fibrinolysis 2003, 14, 261-8. doi: 10.1097/01.mbc.0000061288.28953.c8. PMID: 12695749
- Zavalloni, D.; Lisignoli, V.; Barbaro, C.; Mennuni, M.; Tosi, P.; Marcheselli, S.; Presbitero, P. Platypnoea-orthodeoxia syndrome secondary to patent foramen ovale (PFO): a challenging subset for PFO percutaneous closure. Heart Lung Circ 2013, 22, 642-6. doi: 10.1016/j.hlc.2013.01.007. PMID: 23497825.
- Knapper, J.T.; Schultz, J.; Das, G.; Sperling, L.S. Cardiac platypnea-orthodeoxia syndrome: an often unrecognised malady. Clin Cardiol 2014, 37, 645-9. doi: 10.1002/clc.22301. PMID: 24912004.
- Berger, J.P.; Raveendran, G.; Ingbar, D.H.; Bhargava, M. Hypoxia: an unusual case with specific treatment. Case Rep Pulmonol 2015, 2015, 956341. doi: 10.1155/2015/956341. PMID: 25722910.
- Shah, A.H.; Osten, M.; Leventhal, A.; Bach, Y.; Yoo, D.; Mansour, D.; Benson, L.; Wilson, W.M.; Horlick, E. Percutaneous intervention to treat platypnea-orthodeoxia syndrome: the Toronto experience. JACC Cardiovasc Interv 2016, 9, 1928-38. doi: 10.1016/j.jcin.2016.07.003. PMID: 27659570.
- Mojadidi, M.K.; Ruiz, J.C.; Chertoff, J.; Zaman, M.O.; Elgendy, I.Y.; Mahmoud, A.N.; Al-Ani, M.; Eldendy, A.; Patel, N.K.; Shantha, G.; Tobis, J.M.; Meier, B. Patent foramen ovale and hypoxemia. Cardiol Rev 2019, 27, 34-40. doi: 10.1097/CRD.0000000000000205. PMID: 29570476.
- Nwosu, I.; Ibeson, E.; Singh, S.; Singh, R.; Gulati, A.; Zadushlivy, D.; Kupfer, Y.; Derman, A.; Clemen, B.; Basnet, A.; Nsofor, G.; Ogar, A.U.; Paradoxical thromboembolic ischemic stroke following tissue plasminogen activator instillation for clogged central venous dialysis catheter. Cureus 2021, 13, e20346. doi: 10.7759/cureus.20346. PMID: 35036188.
- Robl, J.; Vutthikraivit, W.; Horwitz, P.; Panaich, S. Percutaneous closure of patent foramen ovale for treatment of hypoxemia: a case series and physiology review. Catheter Cardiovasc Interv 2022, 100, 471-5. doi: 10.1002/ccd.30317. PMID: 35758238.
- Lombardi, M.; Del Buono, M.G.; Princi, G.; Locorotondo, G.; Lombardo, A.; Vergallo, R.; Montone, R.A.; Burzotta, F.; Trani, C.; Crea, F.; Sanna, T.; Platypnoea-orthodeoxia syndrome as an uncommon cause of dyspnoea: a literature review. Intern Med J 2022, 52, 921-5. doi: 10.1111/imj.15669. PMID: 34935270.
- Noori, M.A.M.; Rushdy, A.; Shah, K.K.; Shamoon, F.; Naser, M. Acute-hypoxemia-induced right-to-left shunting in the presence of patent foramen ovale. Cureus 2021, 13, e16138. doi: 10.7759/cureus.16138. PMID: 34262827.
- Sanikommu, V.; Lasorda, D.; Poornima, I.; Anatomical factors triggering platypnea-orthodeoxia in adults. Clin Cardiol 2009, 32, E55-7. doi: 10.1002/clc.20461. PMID: 19816867.
- Abdelsayed, N.; Duff, R.; Faris, M. An unusual case of hypoxia: a case of right-to-left interatrial shunting in a patient with a patent foramen ovale and normal pulmonary pressure. Cureus 2022, 14, e22998. doi: 10.7759/cureus.22998. PMID: 35415050.
- Carvalho, P.; Meireles, D.; Martins, J.L.; Costa, M.; Neves, A.B. An unusual cause of hypoxemia after orthopedic surgery on an elderly patient. Arq Bras Cardiol 2022, 118, 659-62. doi: 10.36660/abc.20210409. PMID: 35319616.
- Arai, N.; Kawachi, R.; Nakazato, Y.; Tachibana, K.; Nagashima, Y.; Tanaka, R.; Okamoto, K.; Kondo, H.; A rare post-lobectomy complication of right-to-left shunt via foramen ovale. Gen Thorac Cardiovasc Surg 2020, 68, 1337-40. doi: 10.1007/s11748-019-01238-9. PMID: 31705454.
- Casanovas-Marbà, N.; Feijoo-Massó, C.; Guillamón-Torán, L.; Guillaumet-Gasa, E.; García-Del Blanco, B.; Martínez-Rubio, A. Patent foramen ovale causing severe hypoxemia due to right-to-left shunting in patients without pulmonary hypertension. Clinical suspicion clues for diagnosis and treatment. Rev Esp Cardiol (Engl Ed) 2014, 67, 324-5. doi: 10.1016/j.rec.2013.09.032. PMID: 24774598.
- Marquetand, C.; Stierle, U.; Buchmann, I.; John, M.; Busch-Tilge, C.; Fuernau, G.; Graf, T.; Kurz, T.; Eitel, I.; Reil, J.C. Severe hypoxemia and stroke caused by a patent foramen ovale with right-to-left interatrial shunt despite normal right atrial pressures. Int J Cardiol Heart Vasc 2021, 34, 100759. doi: 10.1016/j.ijcha.2021.100759. PMID: 33855163.
- Nassif, M.; Lu, H.; Konings, T.C.; Bouma, B.J.; Noordegraaf, A.V.; Straver, B.; Blom, N.A.; Clur, S.A.; Backx, A.P.C.M.; Groenink, M.; Boekholdt, S.M.; Koolbergen, D.R.; Hazekamp, M.G.; Mulder, B.J.M.; de Winter, R.J. Platypnoea-orthodeoxia syndrome, an underdiagnosed cause of hypoxaemia: four cases and the possible underlying mechanisms. Neth Heart J 2015, 23, 539-45. doi: 10.1007/s12471-015-0714-5. PMID: 26170192.
- Ghamande, S.; Ramsey, R.; Rhodes, J.F.; Stoller, J.K. Right hemidiaphragmatic elevation with a right-to-left interatrial shunt through a patent foramen ovale: a case report and literature review. Chest 2001, 120, 2094-6. doi: 10.1378/chest.120.6.2094. PMID: 11742944.
- Strunk, B.L.; Cheitlin, M.D.; Stulbarg, M.S.; Schiller, N.B. Right-to-left interatrial shunting through a patent foramen ovale despite normal intracardiac pressures. Am J Cardiol 1987, 60, 413-5. doi: 10.1016/0002-9149(87)90271-2. PMID: 3618511.
- Laudanski, K.; Patel, S.P.; Peng, Y.G. Ongoing paradoxical particular embolism during megaprosthesis placement. J Clin Anesth 2009, 21, 533-7. doi: 10.1016/j.jclinane.2008.10.018. PMID: 20006264.
- Fisher, D.C.; Fisher, E.A.; Budd, J.H.; Rosen S.E.; Goldman, M.E. The incidence of patent foramen ovale in 1,000 consecutive patients. A contrast transesophageal echocardiography study. Chest 1995, 107, 1504-9. doi: 10.1378/chest.107.6.1504. PMID: 7781337.
- Hagen, P.T.; Scholz, D.G.; Edwards, W.D. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984, 59, 17-20. doi: 10.1016/s0025-6196(12)60336-x. PMID: 6694427.
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Mas, J.L.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; Thomson, J.; Derumeaux, G.; Onorato, E.; Sibbing, D.; Germonpré, P.; Berti, S.; Chessa, M.; Bedogni, F.; Dudek, D.; Hornung, M.; Zamorano, J.; European Association of Percutaneous Cardiovascular Interventions (EAPCI); European Stroke Organisation (ESO); European Heart Rhythm Association (EHRA); European Association for Cardiovascular Imaging (EACVI); Association for European Paediatric and Congenital Cardiology (AEPC); ESC Working group on GUCH; ESC Working group on Thrombosis; European Haematological Society (EHA). European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. EuroIntervention 2019, 14, 1389-1402. doi: 10.4244/EIJ-D-18-00622. PMID: 30141306.
- Vargas-Beal, F.; Coulter, S.A.; Yendamuri, S.; Contreras, A.; Duncan, J.M. Right-to-left interatrial shunt with hypoxemia caused by a right atrial thrombus. Tex Heart Inst J 2007, 34, 225-9. PMID: 17622375.
- Leary, M.C.; Caplan, L.R. Cardioembolic stroke: an update on etiology, diagnosis and management. Ann Indian Acad Neurol 2008, 11, S52-63. PMID: 35721445.
- Homma, S.; Messé, S.R.; Rundek, T.; Sun, Y.P.; Franke, J.; Davidson, K.; Sievert, H.; Sacco, R.L.; Di Tullio, M.R. Patent foramen ovale. Nat Rev Dis Primers 2016, 2, 15086. doi: 10.1038/nrdp.2015.86. PMID: 27188965.
- Hołda, M.K.; Koziej, M. Morphometric features of patent foramen ovale as a risk factor for cerebrovascular accidents: a systematic review and meta-analysis. Cerebrovasc Dis 2020, 49, 1-9. doi: 10.1159/000506433. PMID: 32097931.
- Lamy, C.; Giannesini, C.; Zuber, M.; Arquizan, C.; Meder, J.F.; Trystram, D.; Coste, J.; Mas, J.L. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale; the PFO-ASA study. Atrial septal aneurysm. Stroke 2002, 33, 706-11. doi: 10.1161/hs0302.104543. PMID: 11872892.
- Li, L.; Yiin, G.S.; Geraghty, O.C.; Schulz, U.G.; Kuker, W.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015, 14, 903-13. doi: 10.1016/S1474-4422(15)00132-5. PMID: 26227434.
- Kleber, F.X.; Hauschild, T.; Schulz, A.; Winkelmann, A.; Bruch, L. Epidemiology of myocardial infarction caused by presumed paradoxical embolism via a patent foramen ovale. Circ J 2017, 81, 1484-9. doi: 10.1253/circj.CJ-16-0995. PMID: 28450663.
- Gąsiorek, P.E.; Banach, M.; Maciejewski, M.; Głąbiński, A.; Paduszyńska, A.; Rysz, J.; Bielecka-Dąbrowa, A. Established and potential echocardiographic markers of embolism and their therapeutic implications in patients with ischaemic stroke. Cardiol J 2019, 26, 438-50. doi: 10.5603/CJ.a2018.0046. PMID: 29718528.
- Liu, K.; Wang, B.Z.; Hao, Y.; Song, S.; Pan, M. The correlation between migraine and patent foramen ovale. Front Neurol 2020, 11, 543485. doi: 10.3389/fneur.2020.543485. PMID: 33335507.
- Ioannidis, S.G.; Mitsias, P.D. Patent foramen ovale in cryptogenic ischaemic stroke: direct cause, risk factor, or incidental finding? Front Neurol 2020, 11, 567. doi: 10.3389/fneur.2020.00567. PMID: 32670184.
- Romano, V.; Gallinoro, C.M.; Mottola, R.; Serio, A.; Di Meglio, F.; Castaldo, C.; Sirico, F.; Nurzynska, D. Patent foramen ovale–a not so innocuous septal atrial defect in adults. J Cardiovasc Dev Dis 2021, 8, 60. doi: 10.3390/jcdd8060060. PMID: 34070460.
- Cao, W.; Shen, Y.; Zhong, J.; Chen, Z.; Wang, N.; Yang, J. The patent foramen ovale and migraine: associated mechanisms and perspectives from MRI evidence. Brain Sci 2022, 12, 941. doi: 10.3390/brainsci12070941. PMID: 35884747.
- Travis, J.A.; Fuller, S.B.; Ligush, J.; Plonk Jr, G.W.; Geary, R.L.; Hansen, K.J. Diagnosis and treatment of paradoxical embolus. J Vasc Surg 2001, 34, 860-5. doi: 10.1067/mva.2001.118815. PMID: 11700487.
- Overell, J.R.; Bone, I.; Lees, K.R. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000, 55, 1172-9. doi: 10.1212/wnl.55.8.1172. PMID: 11071496.
- Mattioli, A.V.; Aquilina, M.; Oldani, A.; Longhini, C.; Mattioli, G. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J 2001, 22, 261-8. doi: 10.1053/euhj.2001.2293. PMID: 11161938.
- Handke, M.; Harloff, A.; Olschewski, M.; Hetzel, A.; Geibel, A. Patent foramen ovale and cryptogenic stroke in older patients. N Eng J Med 2007, 357, 2262-8. doi: 10.1056/NEJMoa071422. PMID: 18046029.
- Biteker, M.; Kayataş, K.; Başaran, Ö.; Dogan, V.; Özlek, E.; Özlek, B. The role of left atrial volume index in patients with a first-ever acute ischaemic stroke. J Stroke Cerebrovasc Dis 2017, 26, 321-6. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.023. PMID: 27773589.
- Mojadidi, M.K.; Zaman, M.O.; Elgendy, I.Y.; Mahmoud, A.N.; Patel, N.K.; Agarwal, N.; Tobis, J.M.; Meier, B. Cryptogenic stroke and patent foramen ovale. J Am Coll Cardiol 2018, 71, 1035-43. doi: 10.1016/j.jacc.2017.12.059. PMID: 29495983.
- Al-Sadawi, M.; Madoukh, B.; Battisha, A.; Shaikh, S.; Marmur, J.; Yacoub, F.; McFarlane, S.I. STEMI and CVA in hypercoagulable state with ostium secundum defect. Am J Med Case Rep 2019, 7, 320-4. doi: 10.12691/ajmcr-7-12-5. Epub 2019 Sep 22. PMID: 31650031.
- Rigatelli, G.; Dell’avvocata, F.; Cardaioli, P.; Giordan, M.; Braggion, G.; Aggio, S.; Roncon, L.; Chinaglia, M. Migraine-patient foramen ovale connection: role of prominent eustachian valve and large Chiari network in migrainous patients. Am J Med Sci 2008, 336, 458-61. doi: 10.1097/MAJ.0b013e31816e189d. PMID: 19092317.
- Vale, T.A.; Newton, J.D.; Orchard, E.; Bhindi, R.; Wilson, N.; Ormerod, O.J. Prominence of the Eustachian valve in paradoxical embolism. Eur J Echocardiogr 2011, 12, 33-6. doi: 10.1093/ejechocard/jeq100. PMID: 20813791.
- Jansirani, D.D.; Deep, S.S.; Anandaraja, S. Anatomical study of Chiari network and the remnant of left venous valve in the interior of right atrium. Anat Res Int 2015, 2015, 247680. doi: 10.1155/2015/247680. PMID: 26442159.
- Schuchlenz, H.W.; Saurer, G.; Weihs, W.; Rehak, P. Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr 2004, 17, 231-3. doi: 10.1016/j.echo.2003.12.003. PMID: 14981420.
- Nouh, A.; Hussain, M.; Mehta, T.; Yaghi, S. Embolic strokes of unknown source and cryptogenic stroke: implications in clinical practice. Front Neurol 2016, 7, 37. doi: 10.3389/fneur.2016.00037. PMID: 27047443.
- Homma, S.; Sacco, R.L.; Di Tullio, M.R.; Sciacca, R.R.; Mohr, J.P.; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation 2002, 105, 2625-31. doi: 10.1161/01.cir.0000017498.88393.44. PMID: 12045168.
- Krishnan, S.C.; Salazar, M. Septal pouch in the left atrium: a new anatomical entity with potential for embolic complications. JACC Cardiovasc Interv 2010, 3, 98-104. doi: 10.1016/j.jcin.2009.07.017. PMID: 20129577.
- Wayangankar, S.A.; Patel, J.H.; Patel, B.; Stavrakis, S.; Sivaram, C.A. Clinical and echocardiographic variables associated with LA septal pouch. JACC Cardiovasc Imaging 2013, 6, 833-5. doi: 10.1016/j.jcmg.2012.09.021. PMID: 23664719.
- Paolucci, M.; Vincenzi, C.; Romoli, M.; Amico, G.; Ceccherini, I.; Lattanzi, S.; Bersano, A.; Longoni, M.; Sacco, S.; Vernieri, F.; Pascarella, R.; Valzania, F.; Zedde, M. The genetic landscape of patent foramen ovale: a systematic review. Genes (Basel) 2021, 12, 1953. doi: 10.3390/genes12121953. PMID: 34946902.
- Calcagni, G.; Unolt, M.; Digilio, M.C.; Baban, A.; Versacci, P.; Tartaglia, M.; Baldini, A.; Marino, B. Congenital heart disease and genetic syndromes: new insights into molecular mechanisms. Expert Rev Mol Diagn 2017, 17, 861-70. doi: 10.1080/14737159.2017.1360766. PMID: 28745539.
- Kent, D.M.; Ruthazer, R.; Weimar, C.; Mas, J.L.; Serena, J.; Homma, S.; Di Angelantonio, E.; Di Tullio, M.R.; Lutz, J.S.; Elkind, M.S.V.; Griffith, J.; Jaigobin, C.; Mattle, H.P.; Michel, P.; Mono, M.L.; Nedeltchev, K.; Papetti, F.; Thaler, D.E. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013, 81, 619-25. doi: 10.1212/WNL.0b013e3182a08d59. PMID: 23864310.
- Alyono, J.C. Vertigo and dizziness: understanding and managing fall risk. Otolaryngol Clin North Am 2018, 51, 725-40. doi: 10.1016/j.otc.2018.03.003. PMID: 29803531.
- Whitman, G.T. Dizziness. Am J Med 2018, 131, 1431-7. doi: 10.1016/j.amjmed.2018.05.014. PMID: 29859806.
- Cheng, T.O. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis, and management. Catheter Cardiovasc Interv 1999, 47, 64-6. doi: 10.1002/(SICI)1522-726X(199905)47:1<64::AID-CCD15>3.0.CO;2-6. PMID: 10385164.
- Guérin, P.; Lambert, V.; Godart, F.; Legendre, A.; Petit, J.; Bourlon, F.; De Geeter, B.; Petit, A.; Monrozier, B.; Rossignol, A.M.; Jimenez, M.; Crochet, D.; Choussat, A.; Rey, C.; Losay, J. Transcatheter closure of patent foramen ovale in patients with platypnea-orthodeoxia: results of a multicentric French registry. Cardiovasc Intervent Radiol 2005, 28, 164-8. doi: 10.1007/s00270-004-0035-3. PMID: 15719178.
- Rodrigues, P.; Palma, P.; Sousa-Pereira, L. Platypnea-orthodeoxia syndrome in review: defining a new disease? Cardiology, 2012, 123, 15-23. doi: 10.1159/000339872. PMID: 22948714.
- Agarwal, A.; Palkar, A.; Talwar, A. The multiple dimensions of platypnea-orthodeoxia syndrome: a review. Respir Med 2017, 129, 31-8. doi: 10.1016/j.rmed.2017.05.016. PMID: 28732833.
- Nema, R.; Rajanna, C.; Ray, A.; Jadon, R.S.; Vikram, N.V. When sitting suffocates: a rare cause of platypnoea-orthodeoxia syndrome. Breathe (Sheff) 2020, 16, 200205. doi: 10.1183/20734735.0205-2020. PMID: 33664834.
- Yepes, I.; Ji, S.; Wu, F.; Tijmes, S.; Roberts, J. Platypnea-orthodeoxia syndrome: a rare or under-diagnosed syndrome? 3 case reports and a literature review. Cardiovasc Revasc Med 2021, 22, 115-9. doi: 10.1016/j.carrev.2020.06.002. PMID: 32527601.
- Cao, Q.; Shen, Y.; Hou, Z.; Li, D.; Tang, B.; Xu, L.; Li, Y. The relationship between patent foramen ovale and unexplained dizziness: a prospective analysis in China. Neuropsychiatr Dis Treat 2022, 18, 1495-1505. doi: 10.2147/NDT.S367140. PMID: 35923299.
- Rigatelli, G. Migraine and patent foramen ovale: connecting flight or one-way ticket? Expert Rev Neurother 2008, 8, 1331-7. doi: 10.1586/14737175.8.9.1331. PMID: 18759545.
- Kumar, P.; Kijima, Y.; West, B.H.; Tobis, J.M. The connection between patent foramen ovale and migraine. Neuroimaging Clin N Am 2019, 29, 261-70. doi: 10.1016/j.nic.2019.01.006. PMID: 30926116.
- Lip, P.Z.Y.; Lip, G.Y.H. Patent foramen ovale and migraine attacks: a systematic review. Am J Med 2014, 127, 411-20. doi: 10.1016/j.amjmed.2013.12.006. PMID: 24355354.
- Rudy, C.C.; Ballard, C.; Broberg, C.; Hunter, A.J. Platypnea-orthodeoxia syndrome: a case of chronic paroxysmal hypoxemia. J Gen Intern Med 2017, 32, 127-30. doi: 10.1007/s11606-016-3901-1. PMID: 27785666.
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013, 27, 41-53. doi: 10.1016/j.blre.2012.12.003. PMID: 23291219.
- Johansson, T. Cerebral infarctions with negative CT scans. Eur Neurol 1984, 23, 124-31. doi: 10.1159/000115690. PMID: 6723714.
- Ringelstein, E.B.; Weiller, C. Pattern of cerebral infarct in computerized tomography. Pathophysiologic concepts, validation and clinical relevance. Nervenarzt 1990, 61, 462-71. PMID: 2234222.
- Mullins, M.E.; Schaefer, P.W.; Sorensen, A.G.; Helpern, E.F.; Ay, H.; He, J.; Koroshetz, W.J.; Gonzalez, R.G. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology 2002, 224, 353-60. doi: 10.1148/radiol.2242010873. PMID: 12147827.
- Davis, D.P.; Robertson, T.; Imbesi, S.G. Diffusion-weighted magnetic resonance imaging versus computed tomography in the diagnosis of acute ischaemic stroke. J Emerg Med 2006, 31, 269-77. doi: 10.1016/j.jemermed.2005.10.003. PMID: 16982360.
- Brazzelli, M.; Sandercock, P.A.; Chappell, F.M.; Celani, M.G.; Righetti, E.; Arestis, N.; Wardlaw, J.M.; Deeks, J.J. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev 2009, 4, CD007424. doi: 10.1002/14651858.CD007424.pub2. PMID: 19821415.
- Kucinski, T. Imaging in acute stroke—a personal view. Klin Neuroradiol 2009, 19, 20-30. doi: 10.1007/s00062-009-8030-3. PMID: 19636675.
- Xin, Y.; Han, F.G. Diagnostic accuracy of computed tomography perfusion in patients with acute stroke: a meta-analysis. J Neurol Sci 2016, 360, 125-30. doi: 10.1016/j.jns.2015.11.046. PMID: 26723988.
- Brunser, A.M.; Cavada, G.; Venturelli, P.M.; Olavarría, V.; Rojo, A.; Almeida, J.; Díaz, V.; Hoppe, A.; Lavados, P. Diffusion-weighted imaging determinants for acute ischaemic stroke diagnosis in the emergency room. Neuroradiology 2018, 60, 687-92. doi: 10.1007/s00234-018-2029-x. PMID: 29789895.
- Jadhav, A.P.; Desai, S.M.; Liebeskind, D.S.; Wechsler, L.R. Neuroimaging of acute stroke. Neurol Clin 2020, 38, 185-99. doi: 10.1016/j.ncl.2019.09.004. PMID: 31761058.
- Lin, K.; Do, K.G.; Ong, P.; Shapiro, M.; Babb, J.S.; Siller, K.A.; Pramanik, B.K. Perfusion CT improves diagnostic accuracy for hyperacute ischaemic stroke in the 3-hour window: study of 100 patients with diffusion MRI confirmation. Cerebrovasc Dis 2009, 28, 72-9. doi: 10.1159/000219300. PMID: 19468218.
- Das, T.; Settecase, F.; Boulos, M.; Huynh, T.; d’Esterre, C.D.; Symons, S.P.; Zhang, L.; Aviv, R.I. Multimodal CT provides improved performance for lacunar detection. AJNR Am J Neuroradiol 2015, 36, 1069-75. doi: 10.3174/ajnr.A4255. PMID: 25721075.
- Simonsen, C.Z.; Madsen, M.H.; Schmitz, M.L; Mikkelsen, I.K.; Fisher, M.; Andersen, G. Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke 2015, 46, 98-101. doi: 10.1161/STROKEAHA.114.007107. PMID: 25388415.
- Edlow, B.L.; Hurwitz, S.; Edlow, J.A. Diagnosis of DWI-negative acute ischemic stroke: a meta-analysis. Neurology 2017, 89, 256-62. doi: 10.1212/WNL.0000000000004120. PMID: 28615423.
- Hung, P.; Finn, C.; Chen, M.; Knight-Greenfield, A.; Baradaran, H.; Patel, P.; Díaz, I.; Kamel, H.; Gupta, A. Effect of clinical history on interpretation of computed tomography for acute stroke. Neurohospitalist 2019, 9, 140-3. doi: 10.1177/1941874418825179. PMID: 31244970.
- Zhang, X.H.; Liang, H.M. Systemic review with network meta-analysis: diagnostic values of ultrasonography, computed tomography, and magnetic resonance imaging in patients with ischaemic stroke. Medicine (Baltimore) 2019, 98, e16360. doi: 10.1097/MD.0000000000016360. PMID: 31348236.
- Nagaraja, N. Diffusion weighted imaging in acute ischemic stroke: a review of its interpretation pitfalls and advanced diffusion imaging application. J Neurol Sci 2021, 425, 117435. doi: 10.1016/j.jns.2021.117435. PMID: 33836457.
- Kelley, R.E.; Minagar, A. Cardioembolic stroke: an update. South Med J 2003, 96, 343-9. doi: 10.1097/01.SMJ.0000063471. PMID: 12916551.
- Arboix, A.; Alió, J. Acute cardioembolic cerebral infarction: answers to clinical questions. Curr Cardiol Rev 2012, 8, 54-67. doi: 10.2174/157340312801215791. PMID: 22845816.
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lance Neurol 2014, 13, 429-38. doi: 10.1016/S1474-4422(13)70310-7. PMID: 24646875.
- Ntaios, G.; Papavasileiou, V.; Milionis, H.; Makaritsis, K.; Manios, E.; Spengos, K.; Michel, P.; Vemmos, K. Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis. Stroke 2015, 46, 176-81. doi: 10.1161/STROKEAHA.114.007240. PMID: 25378429.
- Zhang, C.; Kasner, S. Diagnosis, prognosis, and management of cryptogenic stroke. F1000Res 2016, 5, 168. doi: 10.12688/f1000research.7384.1. PMID: 26918178.
- Hart, R.G.; Catanese, L.; Perera, K.S.; Ntaios, G.; Connolly, S.J. Embolic stroke of undetermined source: a systemic review and clinical update. Stroke 2017, 48, 867-72. doi: 10.1161/STROKEAHA.116.016414. PMID: 28265016.
- Ntaios, G. Embolic stroke of undetermined source: JACC review topic of the week. J Am Coll Cardiol 2020, 75, 333-40. doi: 10.1016/j.jacc.2019.11.024. PMID: 31976872.
- Strambo, D.; Sirimarco, G.; Nannoni, S.; Perlepe, K.; Ntaios, G.; Vemmos, K.; Michel, P. Embolic stroke of undetermined source and patent foramen ovale: risk of paradoxical embolism score validation and atrial fibrillation prediction. Stroke 2021, 52, 1643-52. doi: 10.1161/STROKEAHA.120.032453. PMID: 33784832.
- Diener, H.C.; Easton, J.D.; Hart, R.G.; Kasner, S.; Kamel, H.; Ntaios, G. Review and update of the concept of embolic stroke and undetermined source. Nat Rev Neurol 2022, 18, 455-65. doi: 10.1038/s41582-022-00663-4. PMID: 35538232.
- Windecker, S.; Stortecky, S.; Meier, B. Paradoxical embolism. J Am Coll Cardiol 2014, 64, 403-15. doi: 10.1016/j.jacc.2014.04.063. PMID: 25060377.
- He, D.; Shi, Q.; Xu, G.; Hu, Z.; Li, X.; L, Q.; Guo, Y.; Xu, S.; Lin, Y.; Yu, Z.; Wang, W.; Luo, X. Clinical and infarction patterns of PFO-related cryptogenic strokes and a prediction model. Ann Clin Transl Neurol 2018, 5, 1323-37. doi: 10.1002/acn3.647. PMID: 30480027.
- Saver, J.L.; Mattle, H.P.; Thaler, D. Patent foramen ovale closure versus medical therapy for cryptogenic ischaemic stroke: a topical review. Stroke 2018, 49, 1541-8. doi: 10.1161/STROKEAHA.117.018153. PMID: 29760277.
- Alsheikh-Ali, A.A.; Thaler, D.E.; Kent, D.M. Patent foramel ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009, 40, 2349-55. oi: 10.1161/STROKEAHA.109.547828. PMID: 19443800.
- Renard, D.; Ion, I.; Ricci, J.E.; Mura, T.; Thouvenot, E.; Wacongne, A. Chronic small cortical cerebellar infarctions on MRI are associated with patent foramen ovale in young cryptogenic stroke. Cerebrovasc Dis 2020, 49, 105-9. doi: 10.1159/000505959. PMID: 32062661.
- Le Moigne, E.; Timsit, S.; Salem, D.B.; Didier, R.; Jobic, Y.; Paleiron, N.; Le Mao, R.; Joseph, T.; Hoffmann, C.; Dion, A.; Rousset, J.; Le Gal, G.; Lacut, K.; Leroyer, C.; Mottier, D.; Couturaud, F. Patent foramen ovale and ischemic stroke in patients with pulmonary embolism: a prospective cohort study. Ann Intern Med 2019, 170, 756-63. doi: 10.7326/M18-3485. PMID: 31060047.
- Friedrich, S.; Ng, P.Y.; Platzbecker, K.; Burns, S.M.; Banner-Goodspeed, V.; Weimar, C.; Subramanian, B.; Houle, T.T.; Bhatt, D.L.; Eikermann, M. Patent foramen ovale and long-term risk of ischaemic stroke after surgery. Eur Heart J 2019, 40, 914-24. doi: 10.1093/eurheartj/ehy402. PMID: 30020431.
- Mazzucco, S.; Li, L.; Binney, L.; Rothwell, P.M.; Oxford Vascular Study Phenotyped Cohort. Lancet Neurol 2018, 17, 609-17. doi: 10.1016/S1474-4422(18)30167-4. PMID: 29887162.
- Abdelghani, M.; El-Shedoudy, S.A.O.; Nassif, M.; Bouma, B.J.; de Winter, R.J. Management of patients with patent foramen ovale and cryptogenic stroke: an update. Cardiology 2019, 143, 62-72. doi: 10.1159/000501028. PMID: 31307049.
- Kawamura, A.; Lombardi, D.A.; Tilem, M.E.; Gossman, D.E.; Piemonte, T.C.; Nesto, R.W. Stroke complicating percutaneous coronary intervention in patients with acute myocardial infarction. Circ J 2007, 71, 1370-5. doi: 10.1253/circj.71.1370. PMID: 17721013.
- Chong, C.Z.Y.; Tan, B.Y.Q.; Sia, C.H.; Khaing, T.; Yeo, L.L.L. Simultaneous cardiocerebral infarctions: a five-year retrospective case series reviewing natural history. Singapore Med J 2021, online ahead of print. doi: 10.11622/smedj.2021043. PMID: 33866711.
- Hess, D.C.; D’Cruz, I.A.; Adams, R.J.; Nichols 3rd, F.T. Coronary artery disease, myocardial infarction, and brain embolism. Neurol Clin 1993, 11, 399-417. PMID: 8316193.
- Budaj, A.; Flasinska, K.; Gore, J.M.; Anderson Jr, F.A.; Dabbous, O.H.; Spencer, F.A.; Goldberg, R.J.; Fox, K.A.A.; GRACE Investigators. Magnitude of and risk factors for in-hospital and postdischarge stroke in patients with acute coronary syndromes: findings from a Global Registry of Acute Coronary Events. Circulation 2005, 111, 3242-7. doi: 10.1161/CIRCULATIONAHA.104.512806. PMID: 15956123.
- Witt, B.J.; Ballman, K.V.; Brown Jr, R.D.; Meverden, R.A.; Jacobsen, S.J.; Roger, V.L. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006, 119, 354.e1-9. doi: 10.1016/j.amjmed.2005.10.058. PMID: 16564779.
- Saczynski, J.S.; Spencer, F.A.; Gore, J.M.; Gurwitz, J.H.; Yarzebski, J.; Lessard, D.; Goldberg, R.J. Twenty-year trends in the incidence of stroke complicating acute myocardial infarction: Worcester Heart Attack Study. Arch Intern Med 2008, 168, 2104-10. doi: 10.1001/archinte.168.19.2104. PMID: 18955639.
- Hachet, O.; Guenancia, C.; Stamboul, K.; Daubail, B.; Richard, C.; Béjot, Y.; Yameogo, V.; Gudjoncik, A.; Cottin, Y.; Giroud, M.; Lorgis, L. Frequency and predictors of stroke after acute myocardial infarction: specific aspects of in-hospital and postdischarge events. Stroke 2014, 45, 3514-20. doi: 10.1161/STROKEAHA.114.006707. PMID: 25370585.
- Alqahtani, F.; Aljohani, S.; Tarabishy, A.; Busu, T.; Adcock, A.; Alkhouli, M. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke 2017, 48, 2931-8. doi: 10.1161/STROKEAHA.117.018408. PMID: 29018137.
- Bhandari, M.; Vishwakarma, P.; Sethi, R.; Pradhan, A. Stroke complicating acute ST elevation myocardial infarction-current concepts. Int J Angiol 2019, 28, 226-30. doi: 10.1055/s-0039-1695049. PMID: 31787820.
- De Castillo, L.L.; Diestro, J.D.B.; Tuazon, C.A.M.; Sy, M.C.C.; Añonuevo, J.C.; San Jose, M.C.Z. Cardiocerebral infarction: a single institutional series. J Stroke Cerebrovasc Dis 2021, 30, 105831. doi: 10.1016/j.jstrokecerebrovasdis.2021.105831. PMID: 33940364.
- Woo, S.H.; Marhefka, G.D.; Cowan, S.W.; Ackermann, L. Development and validation of a prediction model for stroke, cardiac, and mortality risk after non-cardiac surgery. J Am Heart Assoc 2021, 10, e018013. doi: 10.1161/JAHA.120.018013. PMID: 33522252.
- Bhandari, M.; Pradhan, A.K.; Vishwakarma, P.; Sethi, R. Concurrent coronary, left ventricle, and cerebral thrombosis – a trilogy. Int J Appl Basic Med Res 2022, 12, 130-3. doi: 10.4103/ijabmr.ijabmr_95_21. PMID: 35754676.
- Uddin, M.; Mir, T.; Khalil, A.; Mehar, A.; Gomez-Pineiro, E.; Babu, M.A.; Sheikh, M.; Soubani, A.; Saydain, G.; Afonso, L. J Emerg Med 2022, 62, 306-315. doi: 10.1016/j.jemermed.2021.10.028. PMID: 35058097.
- Yeo, L.L.L.; Andersson, T.; Yee, K.W.; Tan, B.Y.Q.; Paliwal, P.; Gopinathan, A.; Nadarajah, M.; Ting, E.; Teoh, H.L.; Cherian, R.; Lundström, E.; Tay, E.L.W.; Sharma, V.K. Synchronous cardiocerebral infarction in the era of endovascular therapy: which to treat first? J Thromb Thrombolysis 2017, 44, 104-11. doi: 10.1007/s11239-017-1484-2. PMID: 28220330.
- Chin, P.L.; Kaminski, J.; Rout, M. Myocardial infarction coincident with cerebrovascular accidents in the elderly. Age Ageing 1977, 6, 29-37. doi: 10.1093/ageing/6.1.29. PMID: 842403.
- Akinseye, O.A.; Shahreyar, M.; Heckle, M.R.; Khouzam, R.N. Simultaneous acute cardio-cerebral infarction: is there a consensus for management? Ann Transl Med 2018, 6, 7. doi: 10.21037/atm.2017.11.06. PMID: 29404353.
- Gattringer, T.; Niederkorn, K.; Seyfang, L.; Seifert-Held, T.; Simmet, N.; Ferrari, J.; Lang, W.; Brainin, M.; Willeit, J.; Fazekas, F.; Enzinger, C. Myocardial infarction as a complication in acute stroke: results from the Austrian stroke unit registry. Cerebrovasc Dis 2014, 37, 147-52. doi: 10.1159/000357799. PMID: 24481543.
- Mochmann, H.C.; Scheitz, J.F.; Petzold, G.C.; Haeusler, K.G.; Audebert, H.J.; Laufs, U.; Schneider, C.; Landmesser, U.; Werner, N.; Endres, M.; Witzenbichler, B.; Nolte, C.H.; TRELAS Study Group. Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the troponin elevation in acute ischemic stroke (TRELAS) study. Circulation 2016, 133, 1264-71. doi: 10.1161/CIRCULATIONAHA.115.018547. PMID: 26933082.
- Yaghi, S.; Chang, A.D.; Cutting, S.; Jayaraman, M.; McTaggart, R.A.; Ricci, B.A.; Dakay, K.; Narwal, P.; Grory, B.M.; Burton, T.; Reznik, M.; Silver, B.; Gupta, A.; Song, C.; Mehanna, E.; Siket, M.; Lerario, M.P.; Saccetti, D.C.; Merkler, A.E.; Kamel, H.; Elkind, M.S.; Furie, K. Troponin improves the yield of transthoracic echocardiography in ischemic stroke patients of determined stroke subtype. Stroke, 2018, 49, 2777-9. doi: 10.1161/STROKEAHA.118.022477. PMID: 30355193.
- Yaghi, S.; Chang, A.D.; Ricci, B.A.; Jayaraman, M.V.; McTaggart, R.A.; Hemendinger, M.; Narwal, P.; Dakay, K.; Grory, B.M.; Cutting, S.M.; Burton, T.M.; Song, C.; Mehanna, E.; Siket, M.; Madsen, T.E.; Reznik, M.; Merkler, A.E.; Lerario, M.P.; Kamel, H.; Elkind, M.S.V.; Furie, K.L. Early elevated troponin levels after ischemic stroke suggests a cardioembolic source. Stroke 2018, 49, 121-6. doi: 10.1161/STROKEAHA.117.019395. PMID: 29167390.
- Mione, V.; Yao, H.; Laurent, G.; Zeller, M.; Fauchier, L.; Cottin, Y. Simultaneous cardiocerebral embolization in patients with atrial fibrillation. Arch Cardiovasc Dis 2020, 113, 821-7. doi: 10.1016/j.acvd.2020.05.023. PMID: 33153948.
- Zhang, Y.; Ouyang, M.; Qiu, J.; Cao, X.; Xu, B.; Sui, Y. Prognostic value of serum cardiac troponin in acute ischemic stroke: an updated systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2022, 31, 106444. doi: 10.1016/j.jstrokecerebrovasdis.2022.106444. PMID: 35339855.
- Omar, H.R.; Fathy, A.; Rashad, R.; Helal, E. Concomitant acute right ventricular infarction and ischemic cerebrovascular stroke; possible explanations. Int Arch Med 2010, 3, 25. doi: 10.1186/1755-7682-3-25. PMID: 20977759.
- Khechinashvili, G.; Asplund, K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis 2002, 14, 67-76. doi: 10.1159/000064733. PMID: 12187009.
- Grogono, J.; Fitzsimmons, S.J.; Shah, B.N.; Rakhit, D.J.; Gray, H.H. Simultaneous myocardial infarction and ischaemic stroke secondary to paradoxical emboli through a patent foramen ovale. Clin Med (Lond) 2012, 12, 391-2. doi: 10.7861/clinmedicine.12-4-391. PMID: 22930890.
- Abe, S.; Tanaka, K.; Yamagami, H.; Sonoda, K.; Hayashi, H.; Yoneda, S.; Toyoda, K.; Koga, M. Simultaneous cardio-cerebral embolization associated with atrial fibrillation: a case report. BMC Neurol 2019, 19, 152. doi: 10.1186/s12883-019-1388-1. PMID: 31277605.
- Obaid, O.; Smith, H.R.; Brancheau, D. Simultaneous acute anterior ST-elevation myocardial infarction and acute ischemic stroke of left middle cerebral artery: a case report. Am J Case Rep 2019, 20, 776-9. doi: 10.12659/AJCR.916114. PMID: 31154453.
- Ng, T.P.; Wong, C.; Leong, E.L.E.; Tan, B.Y.; Chan, M.Y.Y.; Yeo, L.L.; Yeo, T.C.; Wong, R.C.; Leow, A.S.; Ho, J.S.Y.; Sia, C.H. Simultaneous cardio-cerebral infarction: a meta-analysis. QJM 2022, 115, 374-80. doi: 10.1093/qjmed/hcab158. PMID: 34051098.
- Artus, A.; Didier, R.; Blain, M.; Comby, P.; Leclercq, T.; Debeaumarché, H.; Ricolfi, F.; Zeller, M.; Bejot, Y.; Cochet, A.; Cottin, Y. Detection of myocardial infarction by cardiac magnetic resonance in embolic stroke related to first diagnosed atrial fibrillation. J Stroke Cerebrovasc Dis 2021, 30, 105753. doi: 10.1016/j.jstrokecerebrovasdis.2021.105753. PMID: 33845423.
- Jin, X.; Li, P.; Michalski, D.; Li, S.; Zhang, Y.; Jolkkonen, J.; Cui, L.; Didwischus, N.; Xuan, W.; Boltze, J. Perioperative stroke: a perspective on challenges and opportunities for experimental treatment and diagnostic strategies. CNS Neurosci Ther 2022, 28, 497-509. doi: 10.1111/cns.13816. PMID: 35224865.
- Ibekwe, E.; Kamdar, H.A.; Strohm, T. Cardio-cerebral infarction in left MCA strokes: a case series and literature review. Neurol Sci 2022, 43, 2413-22. doi: 10.1007/s10072-021-05628-x. PMID: 34590206.
- Kim, H.L.; Seo, J.B.; Chung, W.Y.; Zo, J.H.; Kim, M.A.; Kim, S.H. Simultaneously presented acute ischemic stroke and non-ST elevation myocardial infarction in a patient with paroxysmal atrial fibrillation. Korean Circ J 2013, 43, 766-9. doi: 10.4070/kcj.2013.43.11.766. PMID: 24363753.
- Tokuda, K.; Shindo, S.; Yamada, K.; Shirakawa, M.; Uchida, K.; Horimatsu, T.; Ishihara, M.; Yoshimura, S. Acute embolic cerebral infarction and coronary artery embolism in a patient with atrial fibrillation caused by similar thrombi. J Stroke Cerebrovasc Dis 2016, 25, 1797-9. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.055. PMID: 27105568.
- Katsuki, M.; Katsuki, S. A case of cardiac tamponade during the treatment of simultaneous cardio-cerebral infarction associated with atrial fibrillation—case report. Surg Neurol Int 2019, 10, 241. doi: 10.25259/SNI_504_2019. PMID: 31893142.
- Trandafir, C.; Sandiramourty, S.; Laurent-Chabalier, S.; Schiphorst, A.T.; Nguyen, H.; Wacongne, A.; Ricci, J.E.; Pereira, F.; Thouvenot, E.; Renard, D. Brain infarction MRI pattern in stroke patients with intracardiac thrombus. Cerebrovasc Dis 2021, 50, 581-7. doi: 10.1159/000515707. PMID: 34139688.
- Gianstefani, S.; Douiri, A.; Delithanasis, I.; Rogers, T.; Sen, A.; Kalra, S.; Charangwa, L.; Reiken, J.; Monaghan, M.; MacCarthy, P. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014, 113, 1111-6. doi: 10.1016/j.amjcard.2013.12.015. PMID: 24485697.
- Kijpaisalratana, N.; Chutinet, A.; Suwanwela, N.C. Hyperacute simultaneous cardiocerebral infarction: rescuing the brain or the heart first? Front Neurol 2017, 8, 664. doi: 10.3389/fneur.2017.00664. PMID: 29270151.
- Debeaumarché, J.; Thibault, L.; Didier, R.; Debeaumarché, H.; Comby, P.O.; Ricolfi, F.; Zeller, M.; Cochet, A.; Cottin, Y. Acute myocardial infarction related to coronary artery embolism: a systematic cardiac and cerebral magnetic resonance imaging study. Arch Cardiovasc Dis 2022, 115, 457-66. doi: 10.1016/j.acvd.2022.05.005. PMID: 35934614.
- Blanco, M.; Sobrino, T.; Montaner, J.; Medrano, V.; Jiménez, C.; Masjuán, J.; Gómez-Escalonilla, C.; de Luis, P.; Arboix, A.; Castillo, J.; MITICO Study. Stroke with polyvascular atherothrombotic disease. Atherosclerosis 2010, 208, 587-92. doi: 10.1016/j.atherosclerosis.2009.07.041. PMID: 19695570.
- Arboix, A.; Alió. J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev 2010, 6, 150-61. doi: 10.2174/157340310791658730. PMID: 21804774.
- Ay, H.; Koroshetz, W.J.; Benner, T.; Vangel, M.G.; Melinosky, C.; Arsava, E.M.; Ayata, C.; Zhu, M.; Schwamm, L.H.; Sorensen, A.G. Neuroanatomic correlates of stroke-related myocardial injury. Neurology 2006, 66, 1325-9. doi: 10.1212/01.wnl.0000206077.13705.6d. PMID: 16525122.
- Chlapoutakis, G.N.; Kafkas, N.V.; Katsanos, S.M.; Kiriakou, L.G.; Floros, G.V.; Mpampalis, D.K. Acute myocardial infarction and transient ischemic attack in a patient with lone atrial fibrillation and normal coronary arteries. Int J Cardiol 2010, 139, e1-4. doi: 10.1016/j.ijcard.2008.06.085. PMID: 18715661.
- Nagai, M.; Hoshide, S.; Kario, K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens 2010, 4, 174-82. doi: 10.1016/j.jash.2010.05.001. PMID: 20655502.
- Sörös, P.; Hachinski, V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol 2012, 11, 179-88. doi: 10.1016/S1474-4422(11)70291-5. PMID: 22265213.
- Ibrahim, M.S.; Samuel, B.; Mohamed, W.; Suchdev, K. Cardiac dysfunction in neurocritical care: an autonomic perspective. Neurocrit Care 2019, 30, 508-521. doi: 10.1007/s12028-018-0636-3. PMID: 30484009.
- González-Pacheco, H.; Méndez-Domínguez, A.; Vieyra-Herrera, G.; Azar-Manzur, F.; Meave-González, A.; Rodríguez-Zanella, H.; Martínez-Sánchez, C. Reperfusion strategy for simultaneous ST-segment elevation myocardial infarction and acute ischemic stroke within a time window. Am J Emerg Med 2014, 32, 1157. doi: 10.1016/j.ajem.2014.02.047. PMID: 24703066.
- Bersano, A.; Melchiorre, P.; Moschwitis, G.; Tavarini, F.; Cereda, C.; Micieli, G.; Parati, E.; Bassetti, C. Tako-tsubo syndrome as a consequence and cause of stroke. Funct Neurol 2014, 29, 135-7. PMID: 25306124.
- Maciel, R.; Palma, R.; Sousa, P.; Ferreira, F.; Nzwalo, H. Acute stroke with concomitant acute myocardial infarction: will you thrombolyse? J Stroke 2015, 17, 84-6. doi: 10.5853/jos.2015.17.1.84. PMID: 25692111.
- Yang, C.J.; Chen, P.C.; Lin, C.S.; Tsai, C.T.; Tsai, S.H. Thrombolytic therapy-associated acute myocardial infarction in patients with acute ischemic stroke: a treatment dilemma. Am J Emerg Med 2017, 35, 804. doi: 10.1016/j.ajem.2016.11.044. PMID: 27890301.
- Kawano, H.; Tomichi, Y.; Fukae, S.; Koide, Y.; Toda, G.; Yano, K. Aortic dissection associated with acute myocardial infarction and stroke found at autopsy. Intern Med 2006, 45, 957-62. doi: 10.2169/internalmedicine.45.1589. PMID: 16974058.
- Nguyen, T.L.; Rajaratnam, R. Dissecting out the cause: a cause of concurrent acute myocardial infarction and stroke. BMJ Case Rep 2011, 2011, bcr0220113824. doi: 10.1136/bcr.02.2011.3824. PMID: 22693314.
- Yuan, P.J.; Wong, W.K. Acute myocardial infarction and concomitant stroke as the manifestations in a patient with type A aortic dissection: a case report with three years of follow-up. Acta Cardiol Sin 2018, 34, 104-7. doi: 10.6515/ACS.201801_34(1).20170815A. PMID: 29375232.
- Al Adas, Z.; Shepard, A.D.; Weaver, M.R.; Miller, D.J.; Nypaver, T.J.; Modi, S.; Affan, M.; Nour, K.; Balraj, P.; Kabbani, L.S. Cerebrovascular injuries found in acute type B aortic dissections are associated with blood pressure derangements and poor outcome. J Vasc Surg 2018, 68, 1308-13. doi: 10.1016/j.jvs.2018.01.056. PMID: 29945839.
- Mechtouff, L.; Rascle, L.; Crespy, V.; Canet-Soulas, E.; Nighoghossian, N.; Millon, A. A narrative review of the pathophysiology of ischemic stroke in carotid plaques: a distinction versus a compromise between hemodynamic and embolic mechanism. Ann Transl Med 2021, 9, 1208. doi: 10.21037/atm-20-7490. PMID: 34430649.
- Chakir, M.; El Jamili, M.; Boudhar, Z.; El Hattaoui, M. Simultaneous acute myocardial infarction, bilateral pulmonary embolism, and acute ischaemic cerebral stroke, a delayed complication in a patient with COVID-19 infection: case report. Eur Heart J Case Rep 2021, 5, ytab218. doi: 10.1093/ehjcr/ytab218. PMID: 34189400.
- Sakuta, K.; Mukai, T.; Fujii, A.; Makita, K.; Yaguchi, H. Endovascular therapy for concurrent cardio-cerebral infarction in a patient with Trousseau syndrome. Front Neurol 2019, 10, 965. doi: 10.3389/fneur.2019.00965. PMID: 31555206.
- Wang, L.Q.; Mao, X.; Zheng, J.P.; Gu, X.J.; Yang, J.G. A cause of simultaneous acute cardio-cerebral infarction in a woman with essential thrombocythemia. J Int Med Res 2019, 47, 4557-61. doi: 10.1177/0300060519865062. Epub 2019 Aug 20. PMID: 31426696.
- Pezzini, A.; Grassi, M.; Del Zotto, E.; Giossi, A.; Volonghi, I.; Costa, P.; Grau, A.; Magoni, M.; Padovani, A.; Lichy, C. Do common prothrombotic mutations influence the risk of cerebral ischaemia in patients with patent foramen ovale? Systematic review and meta-analysis. Thromb Haemost 2009, 101, 813-7. PMID: 19404532.
- Hviid, C.V.B.; Simonsen, C.Z.; Hvas, A.M. Recurrence risk in patients with cryptogenic stroke, patent foramen ovale, and thrombophilia: a systematic review and meta-analysis. Thromb Haemost 2019, 119, 1839-48. doi: 10.1055/s-0039-1693739. PMID: 31378004.
- Eskandarani, R.; Sahli, S.; Sawan, S.; Alsaeed, A. Simultaneous cardio-cerebral infarction in the coronavirus disease pandemic era: a case series. Medicine (Baltimore) 2021, 100, e24496. doi: 10.1097/MD.0000000000024496. PMID: 33530272.
- Verma, G.C.; Jain, G.; Wahid, A.; Saurabh, C.; Sharma, N.K.; Pathan, A.R.; Ajmera, D. Acute ischaemic stroke and acute myocardial infarction occurring together in domestic low-voltage (220-240V) electrical injury: a rare complication. J Assoc Physicians India 2014, 62, 620-3. PMID: 25672040.
- Sun, R.R.; Chen, T.Z.; Meng. M. Hypereosinophilic syndrome presenting as acute ischemic stroke, myocardial infarction, and arterial involvement: a case report. World J Clin Cases 2022, 10, 3547-52. doi: 10.12998/wjcc.v10.i11.3547. PMID: 35582049.
- Ott, M.C.; Meschia, J.F.; Mackey, D.C.; Brodersen, M.P.; Burger, C.; Echols, J.D.; Fenton, D.S. Cerebral embolization presenting as delayed, severe obtundation in the postanesthesia care unit after total hip arthroplasty. Mayo Clin Proc 2000, 75, 1209-13. doi: 10.4065/75.11.1209. PMID: 11075754.
- Byrick, R.J. Causes of brain injury during orthopedic surgery. Can J Anaesth 2004, 51, 867-70. doi: 10.1007/BF03018881. PMID: 15525609.
- Nikolić, S.; Zivković, V.; Babić, D.; Djonić, D.; Djurić, M. Systemic fat embolism and the patent foramen ovale—a prospective autopsy study. Injury 2012, 43, 608-12. doi: 10.1016/j.injury.2010.08.027. PMID: 20850742.
- Vetrugno, L.; Bignami, E.; Deana, C.; Bassi, F.; Vargas, M.; Orsaria, M.; Bagatto, D.; Intermite, C.; Meroi, F.; Saglietti, F.; Sartori, M.; Orso, D.; Robiony, M.; Bove, T. Cerebral fat embolism after traumatic bone fractures: a structured literature review and analysis of published case reports. Scand J Trauma Resusc Emerg Med 2021, 29, 47. doi: 10.1186/s13049-021-00861-x. PMID: 33712051.
- Giyab, O.; Balogh, B.; Bogner, P.; Gergely, O.; Tóth, A. Microbleeds show a characteristic distribution in cerebral fat embolism. Insights Imaging 2021, 12, 42. doi: 10.1186/s13244-021-00988-6. PMID: 33788069.
- Lempert, M.; Halvachizadeh, S.; Ellanti, P.; Pfeifer, R.; Hax, J.; Jensen, K.O.; Pape, H.C. Incidence of fat embolism syndrome in femur fractures and its associated risk factors over time- a systematic review. J Clin Med 2021, 10, 2733. doi: 10.3390/jcm10122733. PMID: 34205701.
- Bowles, P.F.; Lear, C.; Maccario, M.; Kong, R. Paradoxical air embolism and neurological insult during removal of a pulmonary artery catheter introducer. BMJ Case Rep 2014, 2014, bcr2014203976. doi: 10.1136/bcr-2014-203976. PMID: 25012884.
- Ding, D.Y.; Christoforou, D.; Turner, G.; Tejwani, N.C. Postoperative stroke after hemiarthroplasty for femoral neck fracture: a report of 2 cases and review of literature. J Patient Saf 2014, 10, 117-20. doi: 10.1097/PTS.0000000000000063. PMID: 24618641.
- Hines, C.B. Understanding bone cement implantation syndrome. AANA J 2018, 86, 433-41. PMID: 31584416.
- Christie, J.; Robinson, C.M.; Pell, A.C.; McBirnie, J.; Burnett, R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br 1995, 77, 450-5. PMID: 7744935.
- Lam, P.H.; Milam, A.J.; Ley, E.J.; Yumul, R.; Durra, O. Intraoperative pulmonary embolism diagnosed by rescue transesophageal echocardiography in a morbidly obese patient undergoing orthopedic surgery following motor vehicle crash. Case Rep Anesthesiol 2019, 2019, 2429194. doi: 10.1155/2019/2429194. PMID: 31263602.
- Yeo, L.L.L.; Tan, B.Y.Q.; Andersson, T. Review of post ischemic stroke imaging and its clinical relevance. Eur J Radiol 2017, 96, 145-52. doi: 10.1016/j.ejrad.2017.02.013. PMID: 28237773.
- Moores, M.; Yogendrakumar, V.; Bereznyakova, O.; Alesefir, W.; Pettem, H.; Stotts, G.; Dowlatshahi, D.; Shamy, M. Normal systolic blood pressure at presentation with acute ischemic stroke predicts cardioembolic etiology. J Am Heart Assoc 2020, 9, e014399. doi: 10.1161/JAHA.119.014399. PMID: 31902321.
- Sim, J.E.; Chung, J.W.; Seo, W.K.; Bang, O.Y.; Kim, G.M. Association of systolic blood pressure and cerebral collateral flow in acute ischemic stroke by stroke subtype. Front Neurol 2022, 13, 863483. doi: 10.3389/fneur.2022.863483. PMID: 35645966.
- Tsukazaki, T.; Kuramoto, K.; Oda, S.; Ueda, S.; Matsushita, S. [Myocardial infarction beginning with cerebral symptoms in 30 cases of cardio-cerebral apoplexy]. Nihon Ronen Igakkai Zasshi 1991, 28, 29-33. Article in Japanese. doi: 10.3143/geriatrics.28.29. PMID: 2046162.
- Marto, J.P.; Strambo, D.; Livio, F.; Michel,P. Drugs associated with ischemic stroke: a review for clinicians. Stroke 2021, 52, e646-59. doi: 10.1161/STROKEAHA.120.033272. PMID: 34404236.
- Ranoux, D.; Cohen, A.; Cabanes, L.; Amarenco, P.; Bousser, M.G.; Mas, J.L. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke 1993, 24, 31-4. doi: 10.1161/01.str.24.1.31. PMID: 8418547.
- Schuchlenz, H.W.; Weihs, W.; Horner, S.; Quehenberger, F. The association between the diameter of a patient foramen ovale and the risk of embolic cerebrovascular events. Am J Med 2000, 109, 456-62. doi: 10.1016/s0002-9343(00)00530-1. PMID: 11042234.
- Homma, S.; Sacco, R.L. Patent foramen ovale and stroke. Circulation 2005, 112, 1063-72. doi: 10.1161/CIRCULATIONAHA.104.524371. PMID: 16103257.
- D’Amore, C.; Paciaroni, M. Border-zone and watershed infarctions. Front Neurol Neurosci 2012, 30, 181-4. doi: 10.1159/000333638. PMID: 22377891.
- Yong, S.W.; Bang, O.Y.; Lee, P.H.; Li, W.Y. Internal and cortical border-zone infarctions: clinical and diffusion-weighted imaging features. Stroke 2006, 37, 841-6. doi: 10.1161/01.STR.0000202590.75972.39. PMID: 16424374.
- Mangla, R.; Kolar, B.; Almast, J.; Ekholm, S. E. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics 2011, 31, 1201-14. doi: 10.1148/rg.315105014. PMID: 21918038.
- Cauquil-Michon, C.; Flamand-Roze, C.; Denier, C. Borderzone strokes and transcortical aphasia. Curr Neurol Neurosci Rep 2011, 11, 570-7. doi: 10.1007/s11910-011-0221-z. PMID: 21904919.
- Isabel, C.; Lecler, A.; Turc, G.; Naggara, O.; Schmitt, E.; Belkacem, S.; Oppenheim, C.; Touzé, E. Relationship between watershed infarcts and recent intra plaque haemorrhage in carotid atherosclerotic plaque. PLoS One 2014, 9, e108712. doi: 10.1371/journal.pone.0108712. PMID: 25272160.
- Caplan, L.R.; Hennerici, M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998, 55, 1475-82. doi: 10.1001/archneur.55.11.1475. PMID: 9823834.
- Suter, O.C.; Sunthorn, T.; Kraftsik, R.; Straubel, J.; Darekar, P.; Khalili, K.; Miklossy, J. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke 2002, 33, 1986-92. doi: 10.1161/01.str.0000024523.82311.77. PMID: 12154250.
- Miklossy, J. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer’s disease. Neurol Res 2003, 25, 605-10. doi: 10.1179/016164103101202048. PMID: 14503014.
- Momjian-Mayor, I.; Baron, J.C. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke 2005, 36, 567-77. doi: 10.1161/01.STR.0000155727.82242.e1. PMID: 15692123.
- Hossmann, K.A.; Heiss, W.D. History of the Letzte Wieze/Last Meadon Concept of brain ischemia. Stroke 2016, 47, e46-50. doi: 10.1161/STROKEAHA.115.010976. PMID: 26757752.
- Weill, C.; Suissa, L.; Darcourt, J.; Mahagne, M.H. The pathophysiology of watershed infarction: a three-dimensional time-of-flight magnetic resonance angiography study. J Stroke Cerebrovasc Dis 2017, 26, 1966-73. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.016. PMID: 28694111.
- El-Gammal, T.M.; Bahnasy, W.S.; Ragab, O.A.A.; Al-Malt, A.M. Cerebral border zone infarction: an etiological study. Egypt J Neurol Psychiatr Neurosurg 2018, 54, 6. doi: 10.1186/s41983-018-0008-0. PMID: 29780226.
- Stortecky, S.; da Costa, B.R.; Mattle, H.P.; Carroll, J.; Hornung, M.; Sievert, H.; Trelle, S.; Windecker, S.; Meier, B.; Jüni, P.; Percutaneous closure of patent foramen ovale in patients with cryptogenic embolism: a network meta-analysis. Eur Heart J 2015, 36, 120-8. doi: 10.1093/eurheartj/ehu292. Epub 2014 Aug 11. PMID: 25112661.
- Lee, P.H.; Song, J.K.; Kim, J.S.; Heo, R.; Lee, S.; Kim, D.H.; Song, J.M.; Kang, D.H.; Kwon, S.U.; Kang, D.W.; Lee, D.; Kwon, H.S.; Yun, S.C.; Sun, B.J.; Park, J.H.; Lee, J.H.; Jeong, H.S.; Song, H.J.; Kim, J.; Park, S.J. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol 2018, 71, 2335-42. doi: 10.1016/j.jacc.2018.02.046. PMID: 29544871.
- Sitwala, P.; Khalid, M.F.; Khattak, F.; Bagai, J.; Bhogal, S.; Ladia, V.; Mukherjee, D.; Daggubati, R.; Paul, T.K. Percutaneous closure of patent foramen ovale in patients with cryptogenic stroke – an updated comprehensive meta-analysis. Cardiovasc Revasc Med 2019, 20, 687-94. doi: 10.1016/j.carrev.2018.09.010. PMID: 30282597.
- Diener, H.C.; Grau, A.; Baldus, S. Cryptogenic stroke and patent foramen ovale (abridged and translated version). Neurol Res Pract 2019, 1, 1. doi: 10.1186/s42466-019-0008-2. PMID: 33324867.
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Mas, J.L.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; Thomson, J.; Derumeaux, G.; Onorato, E.; Sibbing, D.; Germonpré, P.; Berti, S.; Chessa, M.; Bedogni, F.; Dudek, D.; Hornung, M.; Zamorano, J.; Evidence Synthesis Team; Eapci Scientific Documents and Initiatives Committee; International Experts. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur Heart J, 2019, 40, 3182-95. doi: 10.1093/eurheartj/ehy649. PMID: 30358849.
- Pristipino, C.; Germonpré, P.; Toni, D.; Sievert, H.; Meier, B.; D’Ascenzo, F.; Berti, S.; Onorato, E.M.; Bedogni, F.; Mas, J.L.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Kyrle, P.A.; Thomson, J.; Derumeaux, G.; Sibbing, D.; Chessa, M.; Hornung, M.; Zamorano, J.; Dudek, D. European position paper on the management of patients with patent foramen ovale. Part II – decompression sickness, migraine, arterial deoxygenation syndromes and select high-risk clinical conditions. EuroIntervention 2021, 17, e367-75. doi: 10.4244/EIJ-D-20-00785. PMID: 33506796.
- Wintzer-Wehekind, J.; Alperi, A.; Houde, C.; Côté, J.M.; Asmarats, L.; Côté, M.; Rodés-Cabau, J. Long-term follow-up after closure of patent foramen ovale in patients with cryptogenic embolism. J Am Coll Cardiol 2019, 73, 278-87. doi: 10.1016/j.jacc.2018.10.061. PMID: 30678757.
- Arboix, A.; Parra, O.; Alió, J. Patent foramen ovale closure in non-lacunar cryptogenic ischemic stroke: where are we now? J Geriatr Cardiol 2021, 18, 67-74. doi: 10.11909/j.issn.1671-5411.2021.01.009. PMID: 33613660.
- Radico, F.; Foglietta, M.; Di Fulvio, M.; Appignani, M.; Rossi, S.; De Angelis, M.V.; Gallina, S.; Zimarino, M. The ‘dreaded PFO’: anatomical and functional features of high risk for stroke. Eur Heart J Suppl 2021, 23, E189-93. doi: 10.1093/eurheartj/suab119. PMID: 35233215.
- Elmariah, S.; Furlan, A.J.; Reisman, M.; Burke, D.; Vardi, M.; Wimmer, N.J.; Ling, S.; Chen, X.; Kent, D.M.; Massaro, J.; Mauri, L.; CLOSURE I Investigators. Predictors of recurrent events in patients with cryptogenic stroke and patent foramen ovale within the CLOSURE I (Evaluation of the STARFlex septal closure system in patients with a stroke and/or transient ischemic attack due to presumed paradoxical embolism through a patent foramen ovale) trial. JACC Cardiovasc Interv 2014, 7, 913-20. doi: 10.1016/j.jcin.2014.01.170. PMID: 25147037.
- Mattle, H.P.; Saver, J.L. Patent foramen ovale increases stroke risk in older people. Nat Rev Neurol 2018, 14, 573-4. doi: 10.1038/s41582-018-0050-7. PMID: 30054555.
- Porter, S.B.; Spaulding, A.C.; Duncan, C.M.; Wilke, B.K.; Pagnano, M.W.; Abdel, M.P. Tranexamic acid was not associated with increased complications in high-risk patients with hip fracture undergoing arthroplasty. J Bone Joint Surg Am 2021, 103, 1880-9. doi: 10.2106/JBJS.21.00172. PMID: 34449443.
- Porter, S.B.; Spaulding, A.C.; Duncan, C.M.; Wilke, B.K.; Pagnano, M.W.; Abdel, M.P. Tranexamic acid was not associated with increased complications in high-risk patients with intertrochanteric fracture. J Bone Joint Surg Am 2022, 104, 1138-47. doi: 10.2106/JBJS.21.01389. PMID: 35775092.
- Zhang, P.; Bai, J.; He, J.; Liang, Y.; Chen, P.; Wang, J. A systematic review of tranexamic acid usage in patients undergoing femoral fracture surgery. Clin Interv Aging 2018, 13, 1579-91. doi: 10.2147/CIA.S163950. PMID: 30233155.
- He, L.; Zhang, R.; Yin, J.; Zhang, H.; Bu, W.; Wang, F.; Zhang, F. Perioperative multidisciplinary implementation enhancing recovery after hip arthroplasty in geriatrics with preoperative chronic hypoxaemia. Sci Rep 2019, 9, 19145. doi: 10.1038/s41598-019-55607-8. PMID: 31844090.
- Qi, Y.M.; Wang, H.P.; Li, Y.J.; Ma, B.B.; Xe, T.; Wang, C.; Chen, H.; Rui, Y.F. The efficacy and safety of intravenous tranexamic acid in hip fracture surgery: a systematic review and meta-analysis. J Orthop Translat 2019, 19, 1-11. doi: 10.1016/j.jot.2019.03.007. PMID: 31844608.
- Haratian, A.; Shelby, T.; Hasan, L.K.; Bolia, I.K.; Weber, A.E.; Petrigliano, F.A. Utilization of tranexamic acid in surgical orthopaedic practice: indications and current considerations. Orthop Res Rev 2021, 13, 187-99. doi: 10.2147/ORR.S321881. PMID: 34703327.
- Kanthasamy, S.; Guhan, B.; Chakravarty, D.; Parker, M.J. The efficacy of intravenous tranexamic acid administration at induction in definitive hip fracture surgery: is there a role? Injury 2021, 52, 2361-6. doi: 10.1016/j.injury.2021.01.032. PMID: 33568279.
- Adams, J.D. Jr; Marshall, W.A. The use of tranexamic acid in hip and pelvic fracture surgeries. J Am Acad Orthop Surg 2021, 29, e576-83. doi: 10.5435/JAAOS-D-20-00750. PMID: 33788803.
- Leverett, G.D.; Marriott, A. Intravenous tranexamic acid and thromboembolic events in hip fracture surgery: a systematic review and meta-analysis. Orthop Traumatol Surg Res 2022, 103337. oi: 10.1016/j.otsr.2022.103337. PMID: 35643364.
- Moran, J.; Kahan, J.B.; Morris, J.; Joo, P.Y.; O’Connor, M.I. Tranexamic acid administration at hospital admission decreases transfusion rates in geriatric hip fracture patients undergoing surgery. Geriatr Orthop Surg Rehabil 2022, 13, 21514593221124414. doi: 10.1177/21514593221124414. PMID: 36081840.
- Jericó-Pascual, I.; Gállego-Cullere, J. [Stroke, tranexamic acid and patent foramen ovale]. Rev Neurol 2008, 46, 186. Article in Spanish. PMID: 18297631.
- Bruce-Brand, R.; Dragomir, R.; Baker, J.; Harty, J. Cerebrovascular infarction following bilateral total knee arthroplasty and tranexamic acid administration. Acta Orthop Belg 2013, 79, 351-4. PMID: 23926741.