Evolutionary clinical-therapeutic study of ocular manifestations in encephalotrigeminal angiomatosis
Marieta Dumitrache*, MD, PhD
Department OF Ophthalmology, “Carol Davila” University of Medicine and Pharmacy, Sibiu, Romania.
*Corresponding author
Marieta Dumitrache, MD, PhD, Department OF Ophthalmology, “Carol Davila” University of Medicine and Pharmacy, Sibiu, Romania.
DOI: 10.55920/JCRMHS.2023.04.001164
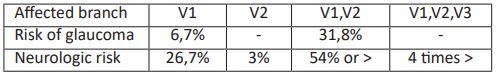
SWS is:
- type 1, affects dermatome v1 – facial, leptomeningeal angioma, glaucoma
- type 2, affectts dermatome v2 – facial angioma and glaucoma
- type 3, affects dermatome v3 – isolated leptomeningeal angioma, absence of glaucoma
SWS is produced by a non-hereditary somatic mosaic mutation in the GNAQ gene, located on chromosome 9q21, before birth. The GNAQ gene is encoded for the Gaq protein, with a role in regulating vascular function; by gene mutation, the embryonic vascular plexus of the neural tube has regression defects (develops in week 6 i.u., regresses in week 9 i.u.) which causes the formation of angiomas, which can be facial or leptomeningeal. (3,4)
Malformations of the fetal vascular system in SWS bring about cortical anoxia, vascular obstructions, ischemia, calcium deposits in the adjacent cortical tissue.
Angiomatous extension and its complications determine the severity of the syndrome, and the severity of clinical manifestations also depend on the time of mutation in fetal development.
Depending on the location of the angiomas, the clinical manifestations in SWS are: cutaneous, neurological, ocular manifestations.
Systemic clinical manifestations in Sturge-Weber Krabbe syndrome
Skin manifestations:
“Port-wine stain” is the most common skin lesion, it is a vascular malformation produced by the dilation of small blood vessels, capillaries, from the surface of the skin, accompanied by venous skin alteration; it is unilateral, with an incidence of 0-3% at birth, of which SWS - 5%.
Port-wine stain is bright red at birth, it increases over time and becomes dark red with hypertrophy and nodules associated with vascular ectasia. Port-wine stain is smooth, raised or flat, isolated or diffuse, sometimes pulsating, over time with a nodular appearance.
The facial plane angioma - Nevus Flamens, present at birth in 76% of cases is a capillary malformation of the hamartoma type, located in the upper part of the face, at the level of the dermatoma innervated by C.N. V – the trigeminal nerve, in particular V1 ophthalmic branch and V2 maxillary branch, with frequently unilateral lesions in 80% of cases. Initially, pink macular lesion that is hyperpigmented and hypertrophied in 60%; it can expand, it can become diffuse, bilateral. Angiomatosis of the facial skin is located in the zygomatic and infraorbital region, it can extend into the nasal cavity, pharynx, oral cavity 70%, sometimes it is associated with angiomas on the body and extremities or it can be associated with macroglossia, oral hemangioma, dental maleruption, maxillary bone hypertrophy. The presence of facial angioma on the eyelids can be a trigger for pathological alterations of the ocular blood flow - 50% of SWS patients have ipsilateral eye pathology, with the facial angioma extended to the eyelids.
Facial angioma can spread and can affect sight, hearing, swallowing. Ocular involvement in SWS is most common in childhood or young adulthood. More than 50% of patients develop glaucoma, on the same side as facial angioma.
Facial angioma requires supervision and treatment if needed; without treatment the angioma tends to grow with the development of bubbles and nodules, with colour changes (darker).
The treatment of facial angioma has questionable results drug treatment is a possible option with uncertain results
- Corticosteroids - triamcinolone injected directly into the angioma systemic, 1mg / kgc, 6 months
- interferon alpha 2a, bleomycin
- for port-wine stain, topical angiogenesis inhibitor to decrease the progression of the lesions
- laser treatment for facial angioma has variable efficacy, depending on the location, size and extent of skin lesions, age of the patient, time of treatment, wavelength, duration of laser application, with greater efficiency before 4 years old
- laser treatment can reduce the partial progression of the angioma cryotherapy associated with laser treatment
Neurological manifestations:
Vascular abnormalities - are venous, leptomeningeal capillary malformations, located in the parietal, occipital area. Neurological symptoms occur later in children or adolescents. Venous stasis produces chronic ischemia with progressive CNS lesions and dystrophic calcifications characteristic of venous cortical calcifications, with parallel arrangement under the form of “train rails” which are calcifications along the meningeal vessels, which are not normally visible.(5)
Leptomeningeal hemangioma is located posteriorly parietally, occipitally with neurological signs of intracranial angiomatosis frequently associated with Jacksonian seizures. Hemangioma can be detected on gadolinium MRI which also shows calcifications.(15)
The main abnormality of leptomeningeal angiomatosis is the defect of normal vein development at the cortical level with the persistence of the primitive fetal complex. Abnormal vessels lead to altered cerebral perfusion and progressive ischemia of the cerebral parenchyma which worsens in the presence of uncontrolled seizures. Vascular malformations become hypoperfused over time by altering venous drainage.
Evaluation of leptomeningeal hemangioma:
CT – subcortical calcifications associated with the decrease in paremchyma volume
- asymmetric enlargement of the cavernous sinus and sinuses
- widening of the ipsilateral choroid plexus
MRI - T1, T2 - calcifications, abnormal venous drainage
Gd – damage to the leptomeningeal area, enlargement of the choroid plexus, dilatation of veins
FA - abnormal, with deeply enlarged venous drainage
Clinically, leptomeningeal angiomatosis is complicated by epilepsy, sometimes refractory to treatment, but also by hemiplegia, hemianopsia, mental retardation.(17)
Epilepsy is the most common neurological complication in SWS, 75-95% of cases; it occurs in the first year of life or at any age (rarely in adults) and may be accompanied by contralateral hemiparesis, hemiplegia, homonymous hemianopsia, a consequence of epilepsy. Epilepsy is common in patients with bialteral leptomeningeal angioma, 95% of cases. In very young children, neurological signs may appear before epileptic seizures: rhythmic spasm of the hand, foot, eyes, followed by tonic-clonic epileptic seizures. The first signs of epilepsy may appear a few weeks after birth - 14%, with initially generalized seizures, most with simple or complex partial seizures, seizures that can generalize with the abolition of consciousness and abnormal motor function during the seizure. In the evolution of the disease, depending on the severity of epilepsy, neurological signs may appear: hemiparesis, contralateral hemianopsia, mental retardation, hemiatrophy. (7,17)
Medical treatment of epilepsy reduces the frequency, severity of seizures and prevents motor and cognitive deficits within severe forms of epilepsy. (6,20)
- phenytoin and barbiturate anticonvulsants are used: carbamazepine, clonazepam, gabapentin, lamotrigen, levetiracetam, phenobarbital, phenytoin, topiramate, valproate
- anticonvulsant treatment can control epilepsy by 40%
- surgical treatment is indicated in epilepsy refractory to anticonvulsant medication by neurosurgical treatment, by partial, total resection, hemispherectomy.
- leptomeningeal hemangioma undergoes a constant vascular reshuffle, with increased VEGF, which can generate apoptosis; antiVEGF treatment may be indicated. (3)
Neuropsychiatric disorders
- mental retardation, the consequence of epileptic seizures
- behavioural disorders with attention deficit, decreased cognitive ability, aggression, depression
- focal neurological deficit: 30% hemiparesis, contralateral hemianopsia due to ischemic dysfunction, hemiplegia, cortical atrophy
- signs of stroke
- headache - 30-45%; migraine that occurs after the epileptic seizure
- the treatment of migraine and headache is done with analgesics and anticonvulsants: triptan, gabapentin, valproic acid, topiramate; aspirin also reduces the risk of thrombosis
Ocular manifestations and therapeutic approach in Sturge-Weber Krabbe syndrome
SWS produces pathological ocular changes ipsilateral to facial angioma in 50% of cases located at the level of: eyelids, conjunctiva, episclera, iridocorneal angle, choroid, retina. Episcleral, trabecular anatomical alterations, Schlemm’s canal in SWS can favour the development of glaucoma which can have two forms: congenital glaucoma - 60% and adult glaucoma - 40%.
Vascular abnormalities of the eyelids, orbit, conjunctiva, episclera, ciliary body, choroid, retina.
Glaucoma is the most common ocular manifestation in SWS (30-70%) with onset at the age of 9,9 +/- 11,9 months, 60% boys (17) and has several clinical forms. (4)
Pathogenically, glaucoma in SWS can be generated by: (16,18)
increased resistance to leakage - mechanically - by malformations of the iridocorneal angle in congenital glaucoma
increase in episleral venous pressure through arteriovenous shunt, in episcleral hemangioma with normal angle structure
hypersecretion of fluid from the ciliary body or choroidal hemangioma
abnormal hemodynamics of the episclera and anterior chamber angle due to premature aging of the trabecular system / Schlemm’s canal in tardive glaucoma (8)
Types of glaucoma in SWS:
early congenital glaucoma (60%) is present at birth and is produced by malformations of the anterior chamber angle with typical signs of congenital primary glaucoma - buphthalmia. It is often associated with corneal changes, with megalocorneal transparency disorders. Regarding the enucleated eyes, patients with SWS showed abnormalities in the iridocorneal angle similar to primary congenital glaucoma: abnormalities of the trabecular meshwork, underdevelopment of the scleral spur, anterior insertion of the iris root. Gonioscopy in congenital glaucoma in SWS highlights the presence of vascular formations in the trabecular meshwork, the presence of giant vacuoles in endothelial cells, extended iris insertion, anterior displacement of the iris root, underdeveloped scleral spur. (9) The incidence of congenital glaucoma increases in parallel with the presence of vascular abnormalities: palpebral, conjunctival, scleral
The pathogenesis of congenital glaucoma in SWS is controversial and sometimes obscure: (11)
- trabeculo-dysgenesis, most commonly
- increase in episcleral venous pressure (EVP) through arteriovenous shunt in the presence of an episcleral hemangioma, homolateral with aqueous humor evacuation disorders, in glaucoma before 2 years old, with increased excavation and discrete bufthalmos. (10) Gonioscopic - increase in EVP shows slow discharge, reflux into the Schlemm’s canal in the eye with glaucoma, often the canal is separated by multiple channels.
- late juvenile glaucoma (40%), present in adolescents, children, occurs by increased EPV with open-angle glaucoma, in the presence of a facial angioma, with trabecular obstruction, by abnormalities in the iridocorneal angle, amplified by choroidal hamgioma, episcleral and / or conjunctival hemangioma that may predispose to glaucoma complications; the risk of glaucoma increases in the presence of palpebral facial angioma
- pathogenically, late glaucoma can be produced by:
- mechanically, in relation to congenital malformations of the iridocorneal angle, with increased leakage resistance
- VPE increase, through arterio-venous shunts and episcleral hemangioma
- abnormal hemodynamics, due to premature aging of the trabecular network, Schlemm canal (5,16)
- open-angle glaucoma in adults can be caused by premature aging of the trabecular system
- secondary glaucoma by neovascularization, rubeosis iridis, anterior synechiae, retinal detachment. (12)
Management of SWS-associated glaucoma is difficult and controversial: medical treatment may be insufficient, and surgical treatment increases the risk of severe complications and should be applied with discernment, especially in the presence of extensive diffuse choroidal hemangioma. (7.11)
Medical and surgical treatment to control intraocular pressure is indicated depending on the pathogenic mechanism, gonioscopy, visual function, IOP, evaluation of the optic nerve, glaucoma progression, disease stage, degree of glaucoma decompensation. (11)
The lack of response to medical treatment requires the indication of surgical treatment with low success rate and possible intraoperative and postoperative surgical hemorrhagic complications.
Early congenital glaucoma associated with angle abnormalities requires surgical treatment, goniotomy or trabeculotomy, with a success rate of up to 60% repeated as needed and indicated in patients under 4 years of age. If necessary, trabeculectomy is indicated.
Goniotomia requires good corneal transparency, an eye with unaltered anatomical structures, with a corneal diameter below 13.5mm; they are most effective between months 2-8.
Trabeculotomy addresses the leakage obstruction of aqueous humor (AH), which is obstructed by a congenital angle abnormality
- is indicated in congenital glaucoma with different degrees of corneal opacification
- controls IOP in 60% postoperatively
Trabeculectomy bypasses the episcleral veins.
- it is indicated when goniotomy and trabeculotomy have failed or there are late alterations of the angle or the treatment is late
Combined technique, trabeculoplasty / trabeculectomy acting on the two mechanisms of glaucoma production in SWS: drainage abnormalities at the iridocorneal angle and increased EPV
Late glaucoma requires an adequate evacuation of intraocular fluid by reducing the leakage resistance of AH, by antiglaucoma medical and / or surgical procedures depending on the type of glaucoma. (6.13)
- Medical treatment is the first line of treatment with beta-blockers and carbonic anhydrase inhibitors (18,19)
- The use of prostagladin analogues should be avoided because they may cause ciliochoroidal effusion in SWS patients.
- Over time, medical treatment will be insufficient and surgical treatment is indicated, but it will be used very carefully for the risk of intraoperative / postoperative haemorrhagic complications: choroidal effusion, choroidal haemorrhagic detachment, recurrent serous choroidal detachment, optical atrophy (10,20,21)
Surgical treatment is indicated if medical treatment does not have an adequate response – trabeculectomy is indicated The surgical treatment indicated in this type of glaucoma if the treatment in glaucoma does not produce an adequate response, is trabeculectomy.
- Trabeculectomy without antimetabolites has a reduced effect in children and young people
- Trabeculectomy with antimetabolites increases the risk of blebitis and bleb-related infection
- Combined technique - trabeculoplasty / trabeculectomy - that acts on the 2 mechanisms of glaucoma, high EPV and drainage abnormalities
- Non-penetrating sclerectomy would have similar efficacy to trabeculectomy but the presence of a possible episcleral hemangioma and angle malformations make the procedure difficult and increase the failure rate
- Laser trabeculoplasty is limited
- Other surgical procedures: valve implant Molteno, Ahmed (14)
- Sometimes, the cryocoagulation of the ciliary body is indicated, associated or not with topical antiglaucoma medication
- Laser cyclophotocoagulation can be indicated in refractory glaucoma in patients at risk of hemorrhagic complications after filtering operations
- Trabeculectomy is associated with an increased risk of uveal infusion and supracoroidal hemorrhage in the SWE eye
- In SWS glaucoma, to reduce the risk of complications that can be extremely serious, the following are necessary:
- Preoperative reduction of intraocular pressure by osmotic agents
- Sealed flap suture with pre-placement of threads
- Use of valves
- If the risk of bleeding complication is related to the presence of choroidal hemangioma, the following are indicated:
- Radiotherapy of choroidal hemangioma before trabeculectomy
- Diode laser transcleral cyclophotocoagulation before the filter operation
- Transpupillary thermotherapy for the partial reduction of diffuse choroidal hemangioma, with diode laser, infrared, with a wavelength of 810nm.
Glaucoma monitoring (5,11,19)
- in children, following the evolution of the anteroposterior axis of the globe, the diameter of the cornea
- gonioscopy - detects malformations from the irido-corneal angle, directs the diagnosis to the type of glaucoma and allows the indication for treatment
- periodic control of IOP, in order to prevent optic nerve damage
- evaluation of the optic nerve, with control of the visual field for diagnosis and monitoring of glaucoma progression
- OCT, a fast non-invasive method for assessing progression in glaucoma, highlights the evolution of the optic nerve head
- oculoorbital ultrasound, mode A, highlights the increase in the anteroposterior axis of the eyeball, the increase in the depth of the anterior chamber
- pachymetry of the cornea (megalocornea – thin cornea)
- slit lamp examination, ophthalmoscopic examination
SWS monitoring
Highlighting and periodic multidisciplinary clinical follow-up of the patient and the disease
- general imaging techniques for diagnosis and evaluation of the severity of lesions, with: skull radiography, cerebral angiography, ultrasound, brain MRI T1, T with contrast, OCT, PET, drug therapeutic monitoring (TDM).
Other eye abnormalities
vascular abnormalities: eyelid, orbit, conjunctiva, episclera, ciliary body, choroid, retina
- episcleral venous plexus
- ampuliform dilatations of the conjunctival vessels
- diffuse or localized choroidal hemangioma
- Heterochromia iridis
Choroidal hemangioma 20-70% is ipsilateral with facial angioma, Choroidal hemangioma is solitary, circumscribed, located in the posterior pole with clear or diffuse boundaries, with a tendency to extension, with toothed edges. Choroidal hemangioma is asymptomatic in children, it can become clinically evident in adolescents, adults, with severe forms, with decreased vision and complications: exudative RD, macular edema, ischemic changes in the optic nerve, if the hemangioma is in its vicinity, with visual field changes , subretinal hemorrhage, serous detachment of the retinal neuroepithelium in the macular area.
Evaluation of hemangioma by:
- Evolutive clinical aspect of the hemangioma
- Ultrasonography - confirms the presence of the lesion, the extension, the echogenicity and the characteristics of the hemangioma
- Indocyanine FA shows the extent of vascular lesions and arteriovenous choroid communications and retinal morphology
- OCT-SD shows the alteration of the retina in the posterior pole, the thickness and morphology of the angioma, evaluates the calibre of vascular and choroidal malformations.
- MRI to determine the thickness of the ocular layers, a consequence of diffuse choroidal hemangioma
The treatment of the hemangioma aims at its involution, the reduction of intra- and subretinal fluid and minimal damage to the neurosensory retina, the treatment being based on the value of visual acuity.
The therapeutic options in hemangioma are:
- confluent hephotocoagulation for tumor destruction
- PDT with risk of scarring and pigmentary changes in the fovea
- transscleral laser diode
- external radiotherapy in diffuse choroidal hemangioma
- brachytherapy cobalt60, ruthenium106
- cyclophotocoagulation, cryotherapy